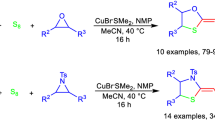
A one-pot methods were developed for the synthesis of five-membered sulfur and selenium heterocycles based on the consecutive cyclometallation of olefins, allenes, and acetylenes using alkyl and haloalkyl derivatives of Al and Mg in the presence of catalytic amounts of titanium and zirconium complexes to give the corresponding alumina- and magnesacarbocycles in situ, which, without further purification, were introduced into reaction with sulfur or selenium, leading to various tetrahydrothiophenes, thiophenes, tetrahydroselenophenes, and selenophenes.
Similar content being viewed by others
References
S. Gronowitz (editor), Thiophene and its Derivatives, Part 1, J. Wiley & Sons, New York (1985), p. 1.
L. I. Belen'kii, E. P. Zakharov, M. A. Kalik, V. P. Litvinov, F. M. Stoyanovich, S. Z. Taits, and B. P. Fabrichnyi, New Directions in Thiophene Chemistry [in Russian], Nauka, Moscow (1976), 424 pp.
L. I. Belen'kii, in: L. I. Belen'kii (editor), Preparation and Properties of Organosulfur Compounds [in Russian], Khimiya, Moscow (1998); p. 344.
Yu. K. Yur'ev, Zh. Obshch. Khim., 6, 1669 (1936).
Belg. Pat. 623801; Chem. Abstr., 59, 8705 (1963).
P. J. Fagan, W. A. Nugent, and J. C. Calabrese, J. Am. Chem. Soc., 116, 1880 (1994).
M. Mizza-Aghayan, R. Boukherroub, G. Etemad-Moghadam, G. Manuel, and M. Koenig, Tetrahedron Lett., 37, 3109 (1996).
M. Zablocka, A. Igau, B. Donnadieu, J. P. Majoral, A. Skowronska, and P. Meunier, J. Chem. Soc., Chem. Commun., 1239 (1997).
Y. Miguel, A. Igau, B. Donnadieu, J. P. Majoral, N. Pirio, and P. Meunier, J. Chem. Soc., Chem. Commun., 279 (1997).
U. M. Dzhemilev and A. G. Ibragimov, J. Organomet. Chem., 466, 1 (1994).
U. M. Dzhemilev and A. G. Ibragimov, Izv. Akad. Nauk, Ser. Khim., 816 (1998).
U. M. Dzhemilev and A. G. Ibragimov, Usp. Khim., 69, 134 (2000).
U. M. Dzhemilev, Tetrahedron, 51, 4333 (1995).
U. M. Dzhemilev and A. G. Ibragimov, Usp. Khim., 74, 886 (2005).
U. M. Dzhemilev, A. G. Ibragimov, A. P. Zolotarev, and G. A. Tolstikov, Izv. Akad. Nauk SSSR, Ser. Khim., 1444 (1989).
U. M. Dzhemilev, A. G. Ibragimov, A. P. Zolotarev, R. R. Muslukhov, and G. A. Tolstikov, Izv. Akad. Nauk SSSR, Ser. Khim., 2831 (1990).
U. M. Dzhemilev, A. G. Ibragimov, A. B. Morozov, R. R. Muslukhov, and G. A. Tolstikov, Izv. Akad. Nauk SSSR, Ser. Khim., 1607 (1991).
U. M. Dzhemilev, A. G. Ibragimov, R. R. Gilyazev, and L. O. Khafizova, Tetrahedron, 60, 1281 (2004).
U. M. Dzhemilev, A. G. Ibragimov, A. P. Zolotarev, L. M. Khalilov, and R. R. Muslukhov, Izv. Akad. Nauk SSSR, Ser. Khim., 386 (1992).
L. O. Khafizova, A. G. Ibragimov, G. N. Gil'fanova, L. M. Khalilov, and U. M. Dzhemilev, Izv. Akad. Nauk, Ser. Khim., 2089 (2001).
A. G. Ibragimov, L. O. Khafizova, G. N. Gil'fanova, and U. M. Dzhemilev, Izv. Akad. Nauk, Ser. Khim., 2095 (2002).
U. M. Dzhemilev, A. G. Ibragimov, L. O. Khafizova, L. P. Yakupova, and L. M. Khalilov, Zh. Org. Khim., 40, 684 (2004).
V. A. D'yakonov, E. Sh. Finkelshtein, and A. G. Ibragimov, Tetrahedron Lett., 48, 8583 (2007).
A. Gordon and R. Ford, Chemist's Companion [Russian translation], Mir, Moscow (1976), p. 301.
V. V. D'yakonov, R. K. Timerkhanov, A. G. Ibragimov, and U. M. Dzhemilev, Izv. Akad. Nauk, Ser. Khim., 2156 (2007).
U. M. Dzhemilev, A. G. Ibragimov, V. A. D'yakonov, and R. A. Zinnurova, Zh. Org. Khim., 43, 184 (2007).
S. T. Ioffe and A. M. Nesmeyanov, Methods in Organoelement Chemistry. Magnesium, Beryllium, Calcium, Strontium, Barium Subgroup [in Russian], Izd. Akad. Nauk SSSR, Moscow (1963); p. 561.
R. Kh. Freidlina, E. M. Brainina, and A. N. Nesmeyanov, Dokl. Akad. Nauk SSSR, 138, 1369 (1969).
P. Cagniant, Bull. Soc. Chim. France, 62 (1953).
Author information
Authors and Affiliations
Corresponding author
Additional information
* Dedicated to Academician B. A. Trofimov of the Russian Academy of Sciences on the occasion of his seventieth jubilee.
Translated from Khimiya Geterotsiklicheskikh Soedinenii, No. 3, pp. 393–403, March, 2009.
Rights and permissions
About this article
Cite this article
D’yakonov, V.A., Ibragimov, A.G., Khalilov, L.M. et al. Dzhemilev reaction in the synthesis of five-membered sulfur and selenium heterocycles*. Chem Heterocycl Comp 45, 317–326 (2009). https://doi.org/10.1007/s10593-009-0272-0
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10593-009-0272-0