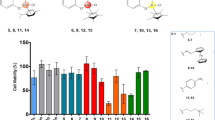
A series of 2-methyl, 4-methyl, and 2,4-dimethyl-8-quinolineselenolates of ruthenium, rhodium, osmium, and iridium has been synthesized and their cytotoxicity towards HT-1080 (human fibrosarcoma) and MG-22A (mouse hepatoma) tumor cells studied. It was found that all of the osmium complexes had a high cytotoxicity towards both cell lines. Their toxicity towards the normal mouse embryonic fibroblasts NIH-3T3 depends on the position and number of methyl groups in the quinoline ring and decreases in the order 2-Me > 4-Me > 2,4-Me2. The greatest selectivity in cytoxic activity is noted for iridium 4-methyl-8-quinolineselenolate and ruthenium 2-methyl-8-quinolineselenolate.
Similar content being viewed by others
References
V. Brabec, J. Kasparkova, in: M. Gielen and E. R. T. Tiekink (editors), Metallotherapeutic Drugs and Metal-Based Diagnostic Agents, J. Wiley and Sons Ltd., Chichester (2005), p. 489.
P. C. Bruijnincx and P. J. Sadler, Current Opinion in Chemical Biology, 12, 197 (2008).
O. Lentzen, C. Mouchenon, and A. Kirsch-De Mesmaeker, in: M. Gielen and E. R. T. Tiekink (editors), Metallotherapeutic Drugs and Metal-Based Diagnostic Agents, J. Wiley and Sons Ltd., Chichester (2005), p. 359.
P. J. Dyson and G. Sava, Dalton Trans., 1929 (2006).
I. Kostova, Curr. Med. Chem., 13, 1085 (2006).
H. A. Wee and P. J. Dyson, Eur. J. Inorg. Chem., 4003 (2006).
E. Meggers, G. E. Atilla-Gokcumen, H. Bregman, J. Maksimoska, S. P. Mulcahy, N. Pagano, and D. S. Williams, Synlett, 1177 (2007).
S. S. Karki, S. Thota, S. Y. Darj, J. Balzarini, and E. De Clercq, Bioorg. Med. Chem., 15, 6632 (2007).
C. A. Vock, W. H. Ang, C. Scolaro, A. D. Phillips, L. Lagopoulos, L. Juillerat-Jeanneret, G. Sava, R. Scopelliti, and P. J. Dyson, J. Med. Chem., 50, 2166 (2007).
M. Auzias, B. Therrien, G. Süss-Fink, P. Štěpnička, H. A. Wee, and P. J. Dyson, Inorg. Chem., 47, 578 (2008).
I. Bratsos, A. Bergamo, G. Sava, T. Gianferrara, E. Zangrando, and E. Alessio, J. Inorg. Biochem., 102, 606 (2008).
I. Bratsos, S. Jedner, A. Bergamo, G. Sava, T. Gianferrara, E. Zangrando, and E. Alessio, J. Inorg. Biochem., 102, 1120 (2008).
S. J. Dougan, A. Habtemariam, S. E. McHale, S. Parsons, and P. J. Sadler, Proc. Natl. Acad. Sci. USA, 105, 11628 (2008).
B. Dutta, C. Scolaro, R. Scopelliti, P. J. Dyson, and K. Severin, Organometallics, 27, 1355 (2008).
A. Garza-Ortiz, P. V. Maheswari, M. Siegler, A. L. Spek, and J. Reedijk, Inorg. Chem., 47, 6964 (2008).
M. Gras, B. Therrien, G. Süss-Fink, P. Štěpnička, A. K. Renfrew, and P. J. Dyson, J. Organomet. Chem., 693, 3419 (2008).
M.-G. Mendoza-Ferri, C. G. Hartinger, R. E. Eichinger, N. Stolyarova, K. Severin, M. A. Jakupec, A. A. Nazarov, and B. K. Keppler, Organometallics, 27, 2405 (2008).
C. Tan, J. Liu, H. Li, W. Zheng, S. Shi, L. Chen, and L. Ji, J. Inorg. Biochem., 102, 347 (2008).
C. Tan, J. Liu, L. Chen, S. Shi, and L. Ji, J. Inorg. Biochem, 102, 1644 (2008).
C. A. Vock, A. K. Renfrew, R. Scopelliti, L. Juillerat-Jeanneret, and P. J. Dyson, Eur. J. Inorg. Chem., 1661 (2008).
F. Schmitt, P. Govindaswamy, G. Süss-Fink, W. H. Ang, P. D. Dyson, L. Juillerat-Jeanneret, and B. Therrien, J. Med. Chem., 51, 1811 (2008).
A. Dorcier, W. H. Ang, S. Bolaño, L. Gonsalvi, L. Juillerat-Jeanneret, G. Laurenzy, M. Peruzzini, A. D. Phillips, F. Zanobini, and P. J. Dyson, Organometallics, 27, 4090 (2006).
F. P. Pruchnik in: M. Gielen and E. R. T. Tiekink (editors), Metallotherapeutic Drugs and Metal-Based Diagnostic Agents, J. Wiley and Sons Ltd., Chichester (2005), p. 379.
D. A. Medvetz, K. D. Stakleff, T. Schreiber, P. D. Custer, K. Hindi, M. J. Panzner, D. D. Blanko, M. J. Tashner, C. A. Tessier, and W. J. Youngs, J. Med. Chem., 50, 1703 (2007).
N. J. Wheate, C. R. Brodie, J. G. Collins, S. Kemp, and J. R. Aldrich-Wright, Mini-Rev. Med. Chem., 7, 627 (2007).
M. Harlos, I. Ott, R. Gust, H. Alborzinia, S. Wölfe, A. Kromm, and W. S. Sheldrick, J. Med. Chem., 51, 3924 (2008).
A. F. A. Peacock, A. Habtemariam, S. A. Moggach, A. Prescimone, S. Parsons, and P. J. Sadler, Inorg. Chem., 46, 4049 (2007).
A. F. A. Peacock, M. Melchart, R. J. Deeth, A. Habtemariam, S. Parsons, and P. J. Sadler, Chem. Eur. J., 13, 2601 (2007).
A. F. A. Peacock, S. Parsons, and P. J. Sadler, J. Am. Chem. Soc., 129, 3348 (2007).
H. Kostrhunova, J. Florian, O. Novakova, A. F. A. Peacock, P. J. Sadler, and V. Brabec, J. Med. Chem., 51, 3635 (2008).
I. N. Stepanenko, A. A. Krokhin, R. O. John, A. Roller, V. B. Arion, M. A. Jakupec, and B. K. Keppler, Inorg. Chem., 47, 7338 (2008).
E. Lukevics, I. Shestakova, I. Domracheva, A. Nesterova, D. Zaruma, and J. Ashaks, Khim. Geterotsikl. Soedin., 870 (2006). [Chem. Heterocycl. Comp., 42, 761 (2006)].
E. Lukevics, I. Shestakova, I. Domracheva, E. Yashchenko, D. Zaruma, and J. Ashaks, Khim. Geterotsikl. Soedin., 755 (2007). [Chem. Heterocycl. Comp., 43, 634 (2007)].
E. Lukevics, D. Zaruma, J. Ashaks, I. Shestakova, I. Domracheva, A. Gulbe, and V. Bridane, Khim. Geterotsikl. Soedin., 711 (2008). [Chem. Heterocycl. Comp., 44, 559 (2008)].
J. Ashaks, Yu. Bankovskii, D. Zaruma, I. Shestakova, I. Domracheva, A. Nesterova, and E. Lukevics, Khim. Geterotsikl. Soedin., 905 (2004). [Chem. Heterocycl. Comp., 40, 776 (2004)].
M. A. Scharwitz, I. Ott, R. Gust, A. Kromm, and W. S. Sheldrick, J. Inorg. Biochem., 102, 1623 (2008).
K. El-Bayoumy, Cancer Res., 45, 3631 (1985).
B. S. Reddy, T. Tanaka, and B. Simi, J. Nat. Cancer. Inst., 75, 791 (1985).
E. Lukevics, P. Arsenyan, K. Rubina, I. Shestakova, I. Domracheva, A. Nesterova, J. Popelis, and O. Pudova, Appl. Organomet. Chem., 16, 235 (2000).
E. Lukevics, P. Arsenyan, I. Shestakova, I. Domracheva, I. Kanepe, S. Belyakov, J. Popelis, and O. Pudova, Appl. Organomet. Chem., 16, 228 (2000).
S. W. May, Expert Opinion on Investigational Drugs, 11, 1261 (2002).
M. Koketsu and H. Ishihara, Curr. Org. Chem., 7, 175 (2003).
A. J. Duffield-Lillico, I. Shureiqi, and S. M. Lippman, J. Nat. Cancer Inst., 96, 1645 (2004).
C. W. Nogueira, G. Zeni, and J. B. T. Rocha, Chem. Rev., 104, 6255 (2004).
M. Soriano-Garcia, Curr. Med. Chem., 11, 1657 (2004).
P. D. Whanger, in: A. S. Award and P. G. Bradford (editors), Nutrition and Cancer Prevention, CRC, Taylor and Francis Group. Boca Raton, London, New York (2006), p. 189.
L. Letavayová, V. Vlčková, and J. Brozmanová, Toxicology, 227, 1 (2006).
G-X. Li, H. Hu, C. Jiang, T. Schuster, and J. Lu, Int. J. Cancer, 120, 2034 (2007).
W. M. El-Sayed, T. Aboul-Fadl, J. C. Roberts, J. G. Lamb, and M. R. Franklin, Toxicology in Vitro, 21, 157 (2007).
W. Mól, M. Matyja, B. Filip, J. Wietrzyk, and S. Boryczka, Bioorg. Med. Chem., 16, 8136 (2008).
E. Lukevics, I. Shestakova, I. Domracheva, A. Nesterova, J. Ashaks, and D. Zaruma, Khim. Geterotsikl. Soedin., 59 (2006). [Chem. Heterocycl. Comp., 42, 53 (2006)].
Guidance Document on Using in vitro Data to Estimate in vivo Starting Doses for Acute Toxicity, National Institute of Health, US Dept. of Health and Human Services (2001), p. 12.
G. Veinberg, M. Vorona, I. Shestakova, I. Kanepe, O. Zharkova, R. Mezapuke, I. Turovskis, I. Kalvinsh, and E. Lukevics, Bioorg. Med. Chem., 8, 1033 (2000).
E. Lukevics, L. Ignatovich, I. Sleiksha, V. Muravenko, I. Shestakova, S. Belyakov, and J. Popelis, Appl. Organometal. Chem., 20, 454 (2006).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Khimiya Geterotsiklicheskikh Soedinenii, No. 2, pp. 230–236, February, 2009.
Rights and permissions
About this article
Cite this article
Lukevics, E., Zaruma, D., Ashaks, J. et al. Synthesis and cytotoxicity of methyl-substituted 8-quinolineselenolates of ruthenium, rhodium, osmium, and iridium. Chem Heterocycl Comp 45, 182–187 (2009). https://doi.org/10.1007/s10593-009-0248-0
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10593-009-0248-0