Abstract
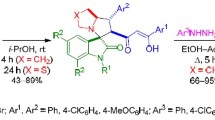
5,11-Disubstituted derivatives of 1′-isopropyl-8-thioxospiro[3,5,7,11-tetrazatricyclo[7.3.1.02,7]tridec-2-ene-13,4′-piperidine]-1,9-dicar bonitrile was obtained by the interaction of 10-amino-9-aza-3-azonia-7,11-dicyano-3-isopropylspiro[5,5]undeca-7,10-diene-8-thiolate with 2 equiv. of a primary amine and excess of formaldehyde. An anomalous reaction product was obtained with o-toluidine — 7,9-dicyano-1′-isopropyl-3-(2-methylphenyl)-1,2,3,4-tetrahydrospiro[pyrido[1,2-a][1,3,5]triazine-8,4′-piper idinium]-6-thiolate.
Similar content being viewed by others
References
V. V. Dotsenko, S. G. Krivokolysko, and V. P. Litvinov, Izv. Acad. Nauk, Ser. Khim., 1418 (2007).
V. P. Litvinov, V. K. Promonenkov, Yu. A. Sharanin, and A. M. Shestopalov, in: Results of Science and Technology. Organic Chemistry. Current Research Directions and Applications of Chemical Preparations in Plant Protection. Chemistry of Azines. [In Russian]. VINITI, Moscow, 1989, Vol. 17, Ch. 2, p.73.
V. P. Litvinov, S. G. Krivokolysko, and V. D. Dyachenko, Khim. Geterotsikl. Soedin., 579 (1999). [Chem. Heterocycl. Comp., 35, 509 (1999)].
V. P. Litvinov, Izv. Akad. Nauk, Ser. Khim., 2123 (1998).
V. P. Litvinov, V. V. Dotsenko, and S. G. Krivokolysko, Izv. Akad. Nauk, Ser. Khim., 847 (2005).
V. P. Litvinov, V. V. Dotsenko, and S. G. Krivokolysko, Chemistry of Thienopyridines and Related Systems [in Russian], Nauka, Moscow, 2006.
E. A. Bakhite, Phosphorus, Sulfur, and Silicon. 178, 929 (2003).
I. M. Orudzheva, T. E. Efendiev, and S. M. Aliev, Zh. Org. Khim., 17, 410 (1981).
Z. Wang, Haoxin Shi, Haijian Shi, Synth. Commun., 31, 2841 (2001).
Z. Wang, T. You, Haijian Shi, Haoxin Shi, Molecules, 1, 89 (1996). Avail URLs: http://www.mdpi.net/molecules/list96.htm; http://www.springerlink.com.
Z. Wang, T. You, Haijian Shi, Haoxin Shi, Gaodeng Xuexiao Huaxue Xuebao, 18, 550 (1997); Chem. Abstr., 127, 95265 (1997).
Hao-Xin Shi, Hai-Jian Shi, Z. Wang, Youji Huaxue, 20, 344 (2000); Chem. Abstr., 133, 120280 (2000).
Z. A. Hozein, A. A. O. Sarhan, H. A. H. El-Sherief, and A. H. Mahmoud, Z. Naturforsch., Teil B, 52, 1401 (1997); Chem. Abstr., 128, 88906 (1998).
Haijian Shi, Haoxin Shi, Z. Wang, J. Heterocycl. Chem., 38, 929 (2001).
Haijian Shi, Z. Wang, Haoxin Shi, Chimia, 51, 529 (1997).
Haijian Shi, Z. Wang, Haoxin Shi, Synth. Commun., 29, 2027 (1999).
S. Yadav Lal Dhar, A. Vaish, and S. Sharma, J. Agr. Food Chem., 42, 811 (1994).
K. A. Frolov, V. V. Dotsenko, S. G. Krivokolysko, and V. I. Litvinov, Izv. Akad. Nauk, Ser. Khim., 2158 (2005).
Z. A. Hozein, J. Chem. Res. (S); No. 3, 99 (2000).
A. O. Sarhan, S. H. Abdel-Hafez, H. El-Sherief, and T. Aboel-Fadl, Synth. Commun., 36, 987 (2006).
V. V. Dotsenko, S. G. Krivokolysko, A. N. Chernega and V. P. Litvinov, Dokl. Akad. Nauk, 389, 763 (2003).
V. V. Dotsenko, Dis. Kand. Chim. Nauk, Moscow (2004).
V. V. Dotsenko, S. G. Krivokolysko, and V. I. Litvinov, Khim. Geterotsikl. Soedin., 1695 (2005).[Chem. Heterocycl. Comp., 41, 1394 (2005)].
V. V. Dotsenko, S. G. Krivokolysko, and V. P. Litvinov, Izv. Akad. Nauk, Ser. Khim., 2605 (2005).
A. A. Shestopalov, Dis. Cand. Chem. Sci., Moscow (2004).
Author information
Authors and Affiliations
Corresponding author
Additional information
__________
Translated from Khimiya Geterotsiklicheskikh Soedinenii, No. 11, 1709–1713, November, 2007.
Rights and permissions
About this article
Cite this article
Dotsenko, V.V., Krivokolysko, S.G. & Litvinov, V.P. The Mannich reaction in the synthesis of N,S-containing heterocycles. 7. Effective one-pot synthesis of derivatives of spiro[3,5,7,11-tetraazatricyclo-[7.3.1.02,7]tridec-2-ene-13,4′-piperidine]. Chem Heterocycl Comp 43, 1455–1459 (2007). https://doi.org/10.1007/s10593-007-0224-5
Received:
Issue Date:
DOI: https://doi.org/10.1007/s10593-007-0224-5