Abstract
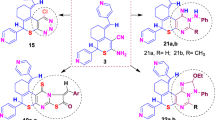
A series of acyclonucleosides 6,7-disubstituted 1-(pent-4-enyl)quinoxalin-2-one derivatives and the O-analogs were synthesized by a one-step condensation of the corresponding quinoxaline bases with 5-bromo-1-pentene.The acyclonucleosides prepared were assayed against HIV-1 and HIV-2 in MT-4 cells. 6,7-Dimethyl-2-(pent-4-enyloxy)quinoxaline showed inhibition of HIV-1 with EC50 value of 0.22 ± 0.08 µg/ml and a therapeutic index of 13. This means that it was cytotoxic to MT-4 cells at CC50 of 2.6 ± 0.1 µg/ml.
Similar content being viewed by others
References
C. K. Chu, S. J. Cutler, J. Heterocycl. Chem., 23, 289 (1986).
R. J. Remy, J. A. Secrist III, Nucleosides Nucleotides, 4, 411 (1985).
T. Miyasaka, H. Tanaka, M. Baba, H. Hayakawa, R. T. Walker, J. Balzarini, E. De Clercq, J. Med. Chem., 32, 2507 (1989).
H. Tanaka, T. Miyasaka, T. Sekiya, H. Takashima, M. Ubasawa, I. Nitta, M. Baba, R. T. Walker, E. De Clercq, Nucleosides Nucleotides, 11, 447 (1992).
G. B. Elion, J. A. Furman, J. A. Fyfe, P. de Miranda, L. Beauchamp, H. Schaeffer, J. Proc. Natl. Acad. Sci. USA, 74, 5716 (1977).
H. J. Schaefer, L. Beauchamp, P. de Miranda, G. B. Elion, D. J. Bauer, P. Collins, Nature (London), 272, 583 (1978).
A. P. Fiddian, D. Brigden, J. M. Yeo, E. A. Hickmott, Antiviral Res., 4, 99 (1984).
C. L. B. Lavelle, J. Oral. Path. Med., 22, 391 (1993).
P. Collins, N. M. Oliver, Antiviral Res., 5, 145 (1985).
P. M. Keller, J. A. Fyfe, L. Beauchamp, C. M. Lubbers, P. A. Furman, H. J. Schaeffer, G. B. Elion, Biochem. Pharmacol., 30, 3071 (1981).
A. Larsson, S. Alenius, N.-G. Johansson, B. Oberg, Antiviral Res., 3, 77 (1983).
A. Holy, Nucleosides Nucleotides, 6, 147 (1987), references cited therein.
J. R. Barrio, J. D. Bryant, G. E. Keyser, J. Med. Chem., 23, 572 (1980).
G. I. Birnbaum, D. Shugar, in: S. Neidle (editor), Nucleic Acids Structure. Pt. 3. Topic in Molecular and Structural Biology, VCH Publishers, New York, 1987, p. 1.
G. W. H. Cheeseman, E. S. G. Werstiuk, Adv. Heterocycl. Chem., 22, 367 (1978).
N. Sato, Comprehensive Heterocyclic Chem. II, Pergamon, Oxford, 1996, vol. 6, p. 233.
K. S. Kim, L. Q. Bird, K. E. Kickinson, S. Moreland, T. R. Schaeffer, T. I. Waldron, D. L. Delany, H. N. Weller, A. V. Miller, J. Med. Chem., 36, 2335 (1993).
R. B. Bandy, L. P. Greenblatt, I. L. Jirkovsky, M. Conklin, R. J. Russo, D. R. Bramlett, T. A. Emrey, J. T. Simmonds, D. M. Kowal, R. P. Stein, R. P. Tasse, J. Med. Chem., 36, 331 (1993).
M. M. Ismail, Y. A. Ammar, M. K. Ibrahim, H. S. El-Zahaby, S. Mahmoud, Arzneim.-Forsch. 55, 738 (2005).
O. S. Moustafa, Y. Yameda, J. Heterocycl. Chem., 38, 809 (2001).
A. V. Bogatskii, S. A. Andronati, Chem. Heterocycl. Comp., 15, 583 (1979).
S. T. Hazeldine, L. Polin, J. Kushner, J. Paluch, K. White, M. Edelstein, E. Palomino, T. H. Corbett, J. P. Horwitz, J. Med. Chem., 44, 1758 (2001).
J. S. Fisherman, B. L. Osborn, H. G. Chun, J. Plowman, A. C. Smith, M. C. Christian, D. S. Zaharko, R. H. Shoemaker, Invest. New Drugs, 11, 1 (1993).
E. S. H. El Ashry, A. A. H. Abdel-Rahman, N. Rashed, H. A. Rasheed, Pharmazie, 54, 893 (1999).
I. A. I. Ali, W. Fathalla, Heteroatom Chem., 17, 280 (2006), references therein.
N. A. Al-Masoudi, Y. A. Al-Soud, M. Eherman, E. De Clercq, Bioog. Med. Chem., 8, 1407 (2000).
N. A. Al-Masoudi, Y. A. Al-Soud, A. Geyer, Tetrahedron, 15, 751 (1999).
N. A. Al-Masoudi, Y. A. Al-Soud, Heteroatom Chem., 15, 380 (2004).
N. A. Al-Masoudi, Y. A. Al-Soud, A. Geyer, Spectroscopy Lett., 31, 1031 (1998).
W. Willker, D. Leibfritz, R. Kerssebaum, W. Bermel, Mag. Reson. Chem., 31, 287 (1993).
A. Bax, R. H. Griffey, B. L. Hawkins, J. Magn. Reson., 55, 301 (1983).
S. Phadtare, J. Zemlicka, J. Med. Chem., 30, 437 (1987).
R. Pauwels, J. Balzarini, M. Baba, R. Snoeck, D. Schols, P. Herdewijn, J. Desmyter, E. De Clercq, J. Virol. Methods, 20, 309 (1988).
S. D. Young, S. F. Britcher, L. O. Tran, L. S. Payne, W. C. Lumma, T. A. Lyle, J. R. Huff, P. S. Anderson, D. B. Olsen, S. S. Carroll, D. J. Pettibone, J. A. Obrien, R. G. Ball, S. K. Balani, J. H. Lin, I. W. Chen, W. A. Schleif, V. V. Sardana, W. J. Long, V. W. Byrnes, E. A. Emini, Antimicrob. Agents Chemother., 39, 2602 (1995).
T. Fujiwara, A. Sato, M. El-Farrash, S. Miki, K. Kabe, Y. Isaka, M. Kodama, Y. M. Wu, L. B. Chen, H. Harada, H. Sugimoto, M. Hatanaka, Y. Hinuma, Y. Antimicrob. Agents Chemother., 42, 1340 (1998).
Author information
Authors and Affiliations
Corresponding author
Additional information
__________
Translated from Khimiya Geterotsiklicheskikh Soedinenii, No. 8, pp. 1243–1250, August, 2007.
Rights and permissions
About this article
Cite this article
Ali, I.A.I., Al-Masoudi, I.A., Hassan, H.G. et al. Synthesis and anti-HIV activity of new homo acyclic nucleosides, 1-(pent-4-enyl)quinoxalin-2-ones and 2-(pent-4-enyloxy)quinoxalines. Chem Heterocycl Comp 43, 1052–1059 (2007). https://doi.org/10.1007/s10593-007-0164-0
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/s10593-007-0164-0