Abstract
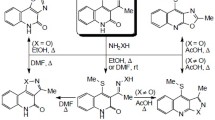
The direction of the reaction of anthranilic acids with o-bromomethylphenylacetonitrile upon fusion depends on the temperature and nature of the substituent in the anthranilic acid. The reaction may lead to three types of products: Derivatives of 7,12-dihydro-5H-isoquino[2,3-a]quinazolin-5-ones below 150°C and to 6,11-dihydro-13H-isoquino[3,2-b]quinazolin-13-one or derivatives of 6H,12H,17H-dibenzo[3,4:6,7][1,8]naphthyridino[1,8-ab]quinazoline-6,17-diones above 150°C depending on the nature of the substituent in the anthranilic acid. A study was carried out on the mechanism for the formation of 6H,12H,17H-dibenzo[3,4:6,7][1,8]naphthyridino[1,8-ab]quinazoline-6,17-diones, which permitted the preparation of 6-(4-methylphenyl)-6,12-dihydro-5H-isoquino[2,3-a]quinazolin-5-one.
Similar content being viewed by others
References
L. M. Potikha, Khim. Geterotsikl. Soedin., 899 (2007). [Chem. Heterocycl. Comp., 43, 759 (2007)].
L. M. Potikha, V. A. Kovtunenko, and V. M. Kisil, Khim. Geterotsikl. Soedin., 562 (2007). [Chem. Heterocycl. Comp., 43, 460 (2007)].
V. M. Kisil, V. A. Kovtunenko, A. V. Turov, A. K. Tyltin, and F. S. Babichev, Dokl. Akad. Nauk, 306, 628 (1989).
V. M. Kisil, L. M. Potikha, R. M. Gutsul, V. A. Kovtunenko, and A. V. Turov, Khim. Geterotsikl. Soedin., 113 (2006). [Chem. Heterocycl. Comp., 42, 100 (2006)].
V. M. Kisil, V. A. Kovtunenko, A. K. Tyltin, and F. S. Babichev, Ukr. Khim. Zh., 56, 749 (1990).
F. S. Babichev, V. K. Patratii, V. A. Kovtunenko, N. G. Prodanchuk, V. G. Zinchenko, and V. M. Kisil, Khim.-farm. Zh., 24, No. 5, 32 (1990).
L. M. Potikha, V. A. Kovtunenko, A. V. Tarasevich, J. G. Wolf, and Ch. André, Khim. Geterotsikl. Soedin., 430 (2007). [Chem. Heterocycl. Comp., 43, 347 (2007)].
C. A. G. Haasnoot, F. A. A. M. de Leeuw, and C. Altona, Tetrahedron, 36, 2783 (1980).
Kh. M. Shakhidoyatov, A. Irisbaev, L. M. Yun, E. Oripov, and Ch. Sh. Kadyrov, Khim. Geterotsikl. Soedin., 1564 (1976). [Chem. Heterocycl. Comp., 12, 1286 (1976)].
L. M. Potikha, N. V. Danileiko, V. M. Kisil, and V. A. Kovtunenko, Khim. Geterotsikl. Soedin., 1214 (2004). [Chem. Heterocycl. Comp., 40, 1052 (2004)].
Author information
Authors and Affiliations
Additional information
__________
Translated from Khimiya Geterotsiklicheskikh Soedinenii, No. 7, pp. 1059–1067, July, 2007.
Rights and permissions
About this article
Cite this article
Potikha, L.M., Kovtunenko, V.A. & Turov, A.V. Condensed isoquinolines 23. Reaction of o-bromomethylphenyl-acetonitrile with anthranilic acids: Synthesis of 6H, 12H,17H-dibenzo[3,4:6,7][1,8]-naphthyridino[1,8-ab]quinazoline-6,17-diones. Chem Heterocycl Compd 43, 893–899 (2007). https://doi.org/10.1007/s10593-007-0141-7
Received:
Issue Date:
DOI: https://doi.org/10.1007/s10593-007-0141-7