Abstract
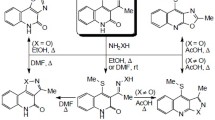
During acylation of 7-isonicotinoyl-6,12-dihydro-5H-isoquino[2,3-a]quinazolin-5-one or its alkylation followed by treatment with organic bases, spirocyclization occurs with formation of derivatives of a novel heterocyclic system: 1′-acyl-and 1′-alkylspiro[7H,12H-6a,11b-diazabenz[e]aceanthrylene-6(5H),4′(1′ H)-pyridine]-5,7-diones. We have studied the spectral properties of the synthesized spirans. We have shown that 7-nicotinoyl-6,12-dihydro-5H-isoquino[2,3-a]quinazolin-5-one does not undergo an analogous reaction of repeated acylation, while treatment of its quaternary salt with bases leads to a complex mixture of unidentified products.
Similar content being viewed by others
References
L. M. Potikha, N. V. Shkilna, V. M. Kisil, and V. A. Kovtunenko, Khim. Geterotsikl. Soedin., 1362 (2004).
V. M. Kisel, V. A. Kovtunenko, L. M. Potikha, A. K. Tyltin, V. S. Nikitchenko, and F. S. Babichev, Ukr. Khim. Zh., 58, 790 (1992).
V. A. Kovtunenko, V. M. Kisil, L. M. Potikha, A. V. Turov, F. S. Babichev, Ukr. Khim. Zh., 59, 1070 (1993).
M. Sainsbury and N. L. Uttely, J. Chem. Soc., Perkin Trans., 2109 (1977).
T. Naito, O. Miyata, and I. Ninomiya, J. Chem. Soc., Chem. Commun., 11, 517 (1979).
T. Naito and I. Ninomiya, Heterocycles, 15, 735 (1981).
J. W. Emsley, J. Feeney, and L. H. Sutcliffe, High Resolution Nuclear Magnetic Resonance Spectroscopy [Russian translation], Mir, Moscow (1968).
P. B. Terentiev and A. P. Stankevicius, Mass Spectroscopic Analysis of Biologically Active Nitrogen Bases [in Russian], Mokslas, Vilnius (1987), p. 86.
D. D. Weller and G. R. Luellen, Tetrahedron Lett., 22, 4381 (1981).
V. M. Kisel, V. A. Kovtunenko, A. V. Turov, A. K. Tyltin, and F. S. Babichev, Dokl. Akad. Nauk, 306, 628 (1989).
V. M. Kisel, L. M. Potikha, A. V. Turov, and V. A. Kovtunenko, Khim. Geterotsikl. Soedin., 1258 (2001).
Author information
Authors and Affiliations
Additional information
For Communication 18, see [1].
__________
Translated from Khimiya Geterotsiklicheskikh Soedinenii, No. 1, pp. 113–122, January, 2006.
Rights and permissions
About this article
Cite this article
Kysil, V.M., Potikha, L.M., Gutsul, R.M. et al. Condensed isoquinolines. 19. Synthesis of 1′-R-spiro[6a,11b-diazabenz[e]aceanthrylene-6(7H),4′ (1′H)-pyridine]-5,7(12H)-diones. Chem Heterocycl Compd 42, 100–108 (2006). https://doi.org/10.1007/s10593-006-0053-y
Received:
Issue Date:
DOI: https://doi.org/10.1007/s10593-006-0053-y