Abstract
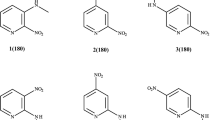
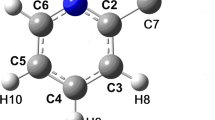
The results of non empirical quantum-chemical calculations using the RHF/6-31G(d) and MP2/6-31G(d) methods do not agree with proposals for the axial position of the H atom on the N atom in the piperidine molecule. According to RHF/6-31G(d) calculations for the N-methylpiperidine molecule and its chloro-substituted derivatives an equatorially placed methyl group is energetically more favored than an axial. The axial C-Cl and C-H bonds in these molecules are longer than the equatorial. The 35 Cl NQR frequencies for the axial Cl atoms are lower than the equatorial. The 35 Cl NQR frequency of the axial chlorine atom in 2-chloro-1-methylpiperidine is anomalously low. This is chiefly due to the high population density of its p σ-orbital and this is a result of the polarization of the C-Cl bond via the N atom unshared electron pair directly through the field. The effect of a similar unshared electron pair on the parameters of the C-Cl bond in the ClCH2NH2 molecule has been studied by the RHF/6-31(g) method for different angles of rotation of the ClCH2 group around the C-N bond.
Similar content being viewed by others
REFERENCES
V. M. Potapov, Stereokhimiya, Khimiya, Moscow (1976), 695 p.
V. P. Feshin, L. I. Zhizhina, and E. V. Feshina, Khim. Geterotsikl. Soedin., 196 (2005).
V. P. Feshin, Electronic Effects in Organic and Organoelemental Molecules [in Russian], Ural Section, Russian Academy of Sciences, Yekaterinburg (1977), 377 p.
V. P. Feshin and E. V. Feshina, Zh. Obshch. Khim., 71, 1895 (2001).
V. P. Feshin and E. V. Feshina, Khim. Geterotsikl. Soedin., 1485 (1999).
V. P. Feshin, E. V. Feshina, and L. I. Zhizhina, Zh. Obshch. Khim., 71, 2004 (2001).
M. J. Frisch, G. W. Trucks, H. B. Schleger, P. M. W. Gill, B. G. Johnson, M. A. Robb, J. R. Cheesman, T. Keith, G. A. Petersson, J. A. Montgomery, K. Raghavachari, M. A. Al-Laham, V. G. Zakrzewski, J. V. Ortiz, J. B. Foresman, J. Cioslowski, B. B. Stefanov, A. Nanayakkara, M. Challacombe, C. Y. Peng, P. Y. Ayala, W. Chen, M. W. Wong, J. L. Andres, E. S. Replogle, R. Gomperts, R. L. Martin, D. J. Fox, J. S. Binkley, D. J. Defrees, J. Baker, J. P. Steward, M. Head-Gordon, C. Gonzalez, and J. A. Pople, Gaussian 94, Revision E. 3, Gaussian Inc, Pittsburgh PA (1995).
T. P. Das and E. L Hahn, Nuclear Quadrupole Resonance Spectroscopy, Academic Press, New York, London (1958), 223 p.
Author information
Authors and Affiliations
Additional information
__________
Translated from Khimiya Geterotsiklicheskikh Soedinenii, No. 7, pp. 1044–1052, July, 2005.
Rights and permissions
About this article
Cite this article
Feshina, E.V., Zhizhina, L.I. & Feshin, V.P. Electronic and Spatial Structure of Piperidine and Its Substituted Derivatives from the Results of ab initio Calculations. Chem Heterocycl Compd 41, 883–889 (2005). https://doi.org/10.1007/s10593-005-0243-z
Received:
Issue Date:
DOI: https://doi.org/10.1007/s10593-005-0243-z