Abstract
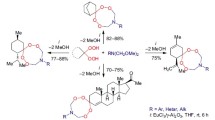
It was shown that isobutyraldehyde and cyanoacetic ester enter into reaction with meta-xylene initially at the fourth carbon atom with the formation of a spiropyrroline ring and then at the newly formed exomethylene bond with closure of the 1-ethoxycarbonylmethylidene-8-(2’-ethoxycarbonylmethylidene-5’,5’-dimethyl-3’-pyrrolidinylidene)-3,3,6-trimethyl-2-azaspiro[4,5]deca-6,9-diene system.
Similar content being viewed by others
REFERENCES
Yu. V. Shklyaev, Yu. V. Nifontov, and V. A. Glushkov, Scientific-Technical Potential of the West Urals in the Region of Conversion of Military-Industrial Complex, Reports of International Seminar [in Russian], Perm (2001), p. 396.
Yu. V. Shklyaev Yu. V. Nifontov (2001) Prospects for Development of Natural Sciences in the High School, Collection of Papers of Scientific Conference 1 63
V. A. Glushkov, Yu. Shklyaev, and V. I. Sokol, Mendeleev Commun., 170 (1999).
Author information
Authors and Affiliations
Additional information
Translated from Khimiya Geterotsiklicheskikh Soedinenii, No. 10, pp. 1487–1491, October, 2004.
Rights and permissions
About this article
Cite this article
Shklyaev, Y.V., Nifontov, Y.V., Kodess, M.I. et al. A novel spiroheterocyclization: synthesis of 1-ethoxycarbonylmethylidene-8-(2’-ethoxycarbonylmethylidene-5’,5’-dimethyl-3’-pyrrolidinylidene)-3,3,6-trimethyl-2-azaspiro[4,5]deca-6,9-diene. Chem Heterocycl Compd 40, 1283–1287 (2004). https://doi.org/10.1007/s10593-005-0056-0
Received:
Issue Date:
DOI: https://doi.org/10.1007/s10593-005-0056-0