Abstract
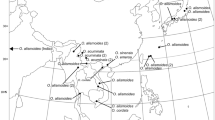
The aquatic lineage consisting of the sister taxa Oxalis dines and O. disticha is confined to a few small vernal pools in the semi-arid Greater Cape Floristic Region of South Africa. All known populations are at risk of extinction due to anthropogenically induced disturbance. To identify priority sites for focused conservation management, the chloroplast intergenic spacer regions psbA-trnH and trnS-trnG were used to determine population structure and genetic diversity in this lineage across its distribution range. Population viability was assessed using flower morph ratios as surrogate for sexual health. Fourteen and four haplotypes were identified from O. disticha and O. dines populations, respectively. Analyses of Molecular Variance indicated an extremely high level of interpopulation differentiation across the entire aquatic lineage as well as within O. disticha and O. dines. Fifty percent of the eighteen haplotypes were confined to single pools, and 84% of populations contained only a single haplotype, even though pool interspacing distance for O. disticha was often less than 5 km. Almost half of O. disticha haplotype diversity was restricted to very small populations. Two O. dines haplotypes were restricted to small populations, with one of these presenting a divergent haplotype sister to the remainder of the aquatic lineage. Flower morph frequency ratio analyses suggested that most populations were reproductively healthy. Low haplotypic diversity within local populations and differentiation between populations are consistent with very low seed-level gene flow and sporadic founder effects. Conservation efforts should be focussed on preserving as many pools as possible with small, genetically distinct populations representing a main concern.

Similar content being viewed by others
References
Google Inc. (2011). Google earth (version 6.0.3.2197) [Software]. Available from http://www.google.com/earth/download/ge/
Balmford A, Mace GM, Ginsberg JR (1998) The challenges to conservation in a changing world: putting processes on the map. In: Mace GM, Balmford A, Ginsberg JR (eds) Conservation in a changing world. Cambridge University Press, Cambridge, pp 1–28
Barrett SCH (1992) Heterostylous genetic polymorphisms: model systems for evolutionary analysis. In: Barrett SCH (ed) Evolution and function of heterostyly, monographs on theoretical and applied genetics, vol 15. Springer-Verlag, Berlin, pp 1–29
Barrett SCH (1993) The evolutionary biology of tristyly. In: Futuyma D, Antonovics J (eds) Oxford surveys in evolutionary biology. Oxford University Press, Oxford, pp 283–326
Beekman M, Ratnieks FLW (2000) Long-range foraging by the honey-bee, Apis mellifera. Funct Ecol 14:490–496
Born J, Linder HP, Desmet P (2007) The greater Cape Floristic region. J Biogeogr 34:147–162
Castro S, Loureiro J, Santos C, Ater M, Ayensa G, Navarro L (2007) Distribution of flower morphs, ploidy level and sexual reproduction of the invasive weed Oxalis pes-caprae in the western area of the Mediterranean region. Ann Bot 99:507–517
Charlesworth D (1979) The evolution and breakdown of tristyly. Evolution 33:486–498
Cowling RM, Pressey RL, Lombard AT, Desmet PG, Ellis AG (1999) From representation to persistence: requirements for a sustainable system of conservation areas in the species-rich Mediterranean-climate desert of southern Africa. Divers Distrib 5:51–71
de Jager ML, Dreyer LL, Ellis AG (2010) Do pollinators influence the assembly of flower colours within plant communities? Oecologia 166:543–553
Dreyer LL (1996) A palynological review of Oxalis (Oxalidaceae) in southern Africa. Ph.D dissertation, University of Pretoria, Pretoria
Eckert CG, Barrett SCH (1992) Stochastic loss of style morphs from populations of Tristylous Lythrum salicaria and Decodon verticillatus (Lythraceae). Evolution 46:1014–1029
Elam D (1998) Population genetics of vernal pool plants: theory data and conservation implications. In: Witham CW, Bauder ET, Belk D, Ferren Jr WR, Ornduff R (eds.) Ecology, conservation and management of vernal pool ecosystems. Proceedings from a 1996 conference. California Native Plant Society, Sacramento, pp 180–189
Excoffier L, Laval G, Schneider S (2005) Arlequin ver. 3.0: an integrated software package for population genetics data analysis. Evol Bioinform Online 1:47–50
Farris JS, Källersjö M, Kluge AG, Bult C (1994) Testing significance of incongruence. Cladistics 10:315–319
Figuerola J, Green AJ (2002) Dispersal of aquatic organisms by waterbirds: a review of past research and priorities for future studies. Freshw Biol 47:483–494
Hall TA (1999) BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Res 41:95–98
Hamilton MB (1999) Four primer pairs for the amplification of chloroplast intergenic regions with intraspecific variation. Mol Ecol 8:521–525
Howe HF, Smallwood J (1982) Ecology of seed dispersal. Ann Rev Ecol Syst 13:201–208
Karron JD (1987) A comparison of levels of genetic polymorphism and self-compatibility in geographically restricted and widespread plant congeners. Evol Ecol 1:47–58
Kass RE, Raftery AE (1995) Bayes factors. J Am Stat Assoc 90:773–795
Keeley JE, Zedler PH (1996) Characterization and global distribution of vernal pools. In: Witham CW, Bauder ET, Belk D, Ferren Jr WR, Ornduff R (eds) Ecology, conservation, and management of vernal pool ecosystems. Proceedings from a 1996 conference. California Native Plant Society, Sacramento, pp 1–14
Lourteig A (2000) Oxalis L. subgenera Monoxalis (Small) Lourteig, Oxalis y Trifidus Lourteig. Bradea 7:201–629
Luo S, Zhang D, Renner SS (2006) Oxalis debilis in China: distribution of flower morphs, sterile pollen and polyploidy. Ann Bot 98:459–464
Millar CI, Libby WJ (1991) Strategies for conserving clinal, ecotypic, and disjunct population diversity in widespread species. In: Falk DA, Holsinger KE (eds) Genetics and conservation of rare plants. Oxford University Press, New York, pp 149–170
Mittermeier RA, Gil PR, Hoffmann M, Pilgrim J, Brooks T, Mittermeier CG, Lamoreux J, da Fonseca GAB (2004) Hotspots revisited: earth’s biologically richest and most endangered ecoregions. CEMEX, Mexico City
Moore J, Picker M (1991) Heuweltjies (earth mounds) in the Clanwilliam District, Cape Province, South Africa: 4000-year-old termite nests. Oecologia 86:424–432
Morgan MT, Barrett SCH (1988) Historical factors and anisoplethic population structure in tristylous Pontederia cordata: a reassessment. Evolution 42:496–504
Moritz C (2002) Strategies to protect biological diversity and the evolutionary processes that sustain it. Syst Biol 51:238–254
Mucina L, Rutherford MC (2006) The vegetation of South Africa, Lesotho and Swaziland. South African National Biodiversity Institute (SANBI), Pretoria
Myers N, Mittermeier RA, Mittermeier CG, da Fonseca GAB, Kent J (2000) Biodiversity hotspots for conservation priorities. Nature 403:853–858
Ness RW, Wright SI, Barrett SCH (2010) Mating-system variation, demographic history and patterns of nucleotide diversity in the tristylous plant Eichhornia paniculata. Genetics 184:381–392
Oberlander KC, Dreyer LL, Bellstedt DU, Reeves G (2004) Systematic relationships in southern African Oxalis L. (Oxalidaceae): congruence between palynological and plastid trnL-F evidence. Taxon 53:977–985
Oberlander KC, Dreyer LL, Curran H (2009) An unusual new species of Oxalis (Oxalidaceae) from the Knersvlakte, South Africa. S Afr J Bot 75:239–245
Oberlander KC, Dreyer LL, Bellstedt DU (2011) Molecular phylogenetics and origins of southern African Oxalis. Taxon 60:1667–1677
Ornduff R (1973) Oxalis dines, a new species from the Western Cape. J S Afr Bot 39:201–203
Ornduff R (1974) Heterostyly in South African flowering plants: a conspectus. J S Afr Bot 40:169–187
Pérez-Alquicira J, Molina-Freaner FE, Piñero D, Weller SG, Martínez-Meyer E, Rozas J, Domínguez CA (2010) The role of historical factors and natural selection in the evolution of breeding systems of Oxalis alpina in the Sonoran desert “Sky Islands”. J Evol Biol 23:2163–2175
Petit RJ, Duminil J, Fineschi S, Hampe A, Salvini D, Vendramin GG (2005) Invited review: comparative organization of chloroplast, mitochondrial and nuclear diversity in plant populations. Mol Ecol 14:689–701
Pons O, Petit RJ (1996) Measuring and testing genetic differentiation with ordered versus unordered alleles. Genetics 144:1237–1245
Posada D (2008) Selection of models of DNA evolution with jModelTest. In: Posada D (ed) Bioinformatics for DNA sequence analysis. Humana Press, Totowa, pp 93–112
Procheş Ş, Cowling RM, Goldblatt P, Manning JC, Snijman DA (2006) An overview of the Cape geophytes. Biol J Linn Soc 87:27–43
Raimondo D, von Staden L, Foden W, Victor JE, Helme NA, Turner RC, Kamundi DA, Manyana PA (2009) Red list of South African plants. South African National Biodiversity Institute (SANBI), Pretoria
Ramp JM, Collinge SK, Ranker TA (2006) Restoration genetics of the vernal pool endemic Lasthenia conjugens (Asteraceae). Conserv Genet 7:631–649
Reed DH, Frankham R (2003) Correlation between fitness and genetic diversity. Conserv Biol 17:230–237
Riggs LA (1990) Conserving genetic resources on-site in forest ecosystems. For Ecol Manag 35:45–68
Ronquist F, Huelsenbeck JP (2003) MrBayes 3: bayesian phylogenetic inference under mixed models. Bioinformatics 19:1572–1574
Salter TM (1944) The genus Oxalis in South Africa: a taxonomic revision. J S African Bot suppl 1:1–355
Simmons MP, Ochoterena H (2000) Gaps as characters in sequence-based phylogenetic analyses. Syst Biol 49:369–381
Sloop CM, Pickens C, Gordon SP (2010) Conservation genetics of Butte County meadowfoam (Limnanthes floccosa ssp. californica Arroyo), an endangered vernal pool endemic. Conserv Genet 12:311–323
Sokal RR, Rohlf FJ (1995) Biometry: the principles and practice of statistics in biological research. W. H. Freeman Publishers, New York
Swofford DL (2002) Phylogenetic analysis using parsimony (*and other methods). Version 4. Sinauer Associates, Sunderland
Verboom GA, Archibald JK, Bakker FT, Bellstedt DU, Conrad F, Dreyer LL, Forest F, Galley C, Goldblatt P, Henning JF et al (2009) Origin and diversification of the Greater Cape flora: ancient species repository, hot-bed of recent radiation, or both? Mol Phylogenet Evol 51:44–53
Weller SG, Domínguez CA, Molina-Freaner FE, Fornoni J, LeBuhn G (2007) The evolution of distyly from tristyly in populations of Oxalis alpina (Oxalidaceae) in the Sky Islands of the Sonoran Desert. Am J Bot 94:972–985
Zietsman J, Dreyer LL, Van Vuuren BJ (2009) Genetic differentiation in Oxalis (Oxalidaceae): a tale of rarity and abundance in the Cape Floristic Region. S Afr J Bot 75:27–33
Acknowledgments
We thank J. Suda for collecting samples of O. namaquana, and A. J. Rebelo for assistance with preparing the figure. This work was supported by a National Research Foundation grant to L. Dreyer (GUN no. 2053585).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Oberlander, K.C., Roets, F. & Dreyer, L.L. Chloroplast phylogeography of threatened aquatic Oxalis (Oxalidaceae): significant inter-population structure, divergent haplotypes and conservation implications. Conserv Genet 13, 789–799 (2012). https://doi.org/10.1007/s10592-012-0329-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10592-012-0329-y