Abstract
Breast cancer is one of the most prevalent malignancies in women, and approximately 75–80% of patients with advanced breast cancer develop bone metastasis. Expression of the cancer-associated carbohydrate antigen sialyl-Tn (STn) in breast cancer is associated with a poor prognosis; however, involvement of STn in the development of metastatic bone lesions remains unclear. We investigated whether STn expression on breast cancer cells influences intraosseous tumor growth and bone response in mice models of skeletal colonization. STn-positive (STn+) breast cancer cells were generated by stable transfection of an expression vector encoding ST6GaLNAc I into the breast cancer cell line MDA-MB-231. Parental MDA-MB-231 cells not expressing STn antigen were used as STn-negative (STn−) breast cancer cells. Contrary to expectations, STn expression attenuated the development of destructive bone lesions in the in vivo mice models. An in vitro study demonstrated that STn expression impaired adhesion of MDA-MB-231cells to bone marrow stromal cells. This finding in vitro was also confirmed by another breast cancer cell line MCF-7. Cell adhesion to fibronectin and type I collagen was also impaired in STn+ MDA-MB-231 cells compared to that in STn− MDA-MB-231 cells, suggesting integrin dysfunction. Given that the integrin β1 subunit is the main carrier of the STn epitope, it is likely that changes in glycan structure impaired the adhesive capacity of β1 integrin in the bone environment, leading to attenuation of tumor cell engraftment. In conclusion, breast cancer cells expressing STn antigen had less capacity for skeletal colonization, possibly due to impaired adhesive capability.






Similar content being viewed by others
References
Setiawan VW et al (2009) Breast cancer risk factors defined by estrogen and progesterone receptor status: the multiethnic cohort study. Am J Epidemiol 169(10):1251–1259
Boyce BF, Yoneda T, Guise TA (1999) Factors regulating the growth of metastatic cancer in bone. Endocr Relat Cancer 6(3):333–347
Roodman GD (2004) Mechanisms of bone metastasis. Discov Med 4(22):144–148
Yong M et al (2011) Survival in breast cancer patients with bone metastases and skeletal-related events: a population-based cohort study in Denmark (1999-2007). Breast Cancer Res Treat 129(2):495–503
Ikehara Y et al (1999) Cloning and expression of a human gene encoding an N-acetylgalactosamine-alpha2,6-sialyltransferase (ST6GalNAc I): a candidate for synthesis of cancer-associated sialyl-Tn antigens. Glycobiology 9(11):1213–1224
Kinney AY et al (1997) The prognostic significance of sialyl-Tn antigen in women treated with breast carcinoma treated with adjuvant chemotherapy. Cancer 80(12):2240–2249
Ma XC et al (1993) Expression of sialyl-Tn antigen is correlated with survival time of patients with gastric carcinomas. Eur J Cancer 29A(13):1820–1823
Werther JL et al (1994) Mucin-associated sialosyl-Tn antigen expression in gastric cancer correlates with an adverse outcome. Br J Cancer 69(3):613–616
Werther JL et al (1996) Sialosyl-Tn antigen as a marker of gastric cancer progression: an international study. Int J Cancer 69(3):193–199
Itzkowitz SH et al (1990) Sialosyl-Tn. A novel mucin antigen associated with prognosis in colorectal cancer patients. Cancer 66(9):1960–1966
Ghazizadeh M et al (1997) Mucin carbohydrate antigens (T, Tn, and sialyl-Tn) in human ovarian carcinomas: relationship with histopathology and prognosis. Hum Pathol 28(8):960–966
Kobayashi H, Terao T, Kawashima Y (1992) Serum sialyl Tn as an independent predictor of poor prognosis in patients with epithelial ovarian cancer. J Clin Oncol 10(1):95–101
Cho SH et al (1994) Sialyl-Tn antigen expression occurs early during human mammary carcinogenesis and is associated with high nuclear grade and aneuploidy. Cancer Res 54(24):6302–6305
Leivonen M et al (2001) STn and prognosis in breast cancer. Oncology 61(4):299–305
Radhakrishnan P et al (2014) Immature truncated O-glycophenotype of cancer directly induces oncogenic features. Proc Natl Acad Sci USA 111(39):E4066–E4075
Munkley J (2016) The role of Sialyl-Tn in cancer. Int J Mol Sci 17(3):275
Angata T et al (2007) Siglec-15: an immune system Siglec conserved throughout vertebrate evolution. Glycobiology 17(8):838–846
Takahata M et al (2007) Sialylation of cell surface glycoconjugates is essential for osteoclastogenesis. Bone 41(1):77–86
Flavell RA et al (2010) The polarization of immune cells in the tumour environment by TGFbeta. Nat Rev Immunol 10(8):554–567
Takamiya R et al (2013) The interaction between Siglec-15 and tumor-associated sialyl-Tn antigen enhances TGF-β secretion from monocytes/macrophages through the DAP12-Syk pathway. Glycobiology 23(2):178–187
Kuchimaru T et al (2018) A reliable murine model of bone metastasis by injecting cancer cells through caudal arteries. Nat Commun 9(1):2981
Mirels H (1989) Metastatic disease in long bones. A proposed scoring system for diagnosing impending pathologic fractures. Clin Orthop Relat Res 249:256–264
Takeshita S, Kaji K, Kudo A (2000) Identification and characterization of the new osteoclast progenitor with macrophage phenotypes being able to differentiate into mature osteoclasts. J Bone Miner Res 15(8):1477–1488
Chen Y et al (2009) Combined integrin phosphoproteomic analyses and small interfering RNA–based functional screening identify key regulators for cancer cell adhesion and migration. Cancer Res 69(8):3713–3720
Munkley J et al (2015) The androgen receptor controls expression of the cancer-associated sTn antigen and cell adhesion through induction of ST6GalNAc1 in prostate cancer. Oncotarget 6(33):34358–34374
Carmona-Fontaine C et al (2011) Complement fragment C3a controls mutual cell attraction during collective cell migration. Dev Cell 21(6):1026–1037
Peister A et al (2004) Adult stem cells from bone marrow (MSCs) isolated from different strains of inbred mice vary in surface epitopes, rates of proliferation, and differentiation potential. Blood 103(5):1662–1668
Clément M et al (2004) Expression of sialyl-Tn epitopes on beta1 integrin alters epithelial cell phenotype, proliferation and haptotaxis. J Cell Sci 117(Pt 21):5059–5069
Guise TA et al (2006) Basic mechanisms responsible for osteolytic and osteoblastic bone metastases. Clin Cancer Res 12(20 Pt 2):6213s–6216s
Ozaki H et al (2012) Enhancement of metastatic ability by ectopic expression of ST6GalNAcI on a gastric cancer cell line in a mouse model. Clin Exp Metastasis 29(3):229–238
Julien S et al (2006) ST6GalNAc I expression in MDA-MB-231 breast cancer cells greatly modifies their O-glycosylation pattern and enhances their tumourigenicity. Glycobiology 16(1):54–64
Liesveld JL, Dipersio JF, Abboud CN (1994) Integrins and adhesive receptors in normal and leukemic CD34 + progenitor cells: potential regulatory checkpoints for cellular traffic. Leuk Lymphoma 14(1–2):19–28
Korah R, Boots M, Wieder R (2004) Integrin alpha5beta1 promotes survival of growth-arrested breast cancer cells: an in vitro paradigm for breast cancer dormancy in bone marrow. Cancer Res 64(13):4514–4522
Martin-Thouvenin V et al (1992) Phorbol ester-induced promyelocytic leukemia cell adhesion to marrow stromal cells involves fibronectin specific alpha 5 beta 1 integrin receptors. J Cell Physiol 153(1):95–102
Michigami T et al (2000) Cell-cell contact between marrow stromal cells and myeloma cells via VCAM-1 and alpha(4)beta(1)-integrin enhances production of osteoclast-stimulating activity. Blood 96(5):1953–1960
Mori Y et al (2004) Anti-alpha4 integrin antibody suppresses the development of multiple myeloma and associated osteoclastic osteolysis. Blood 104(7):2149–2154
Van der Velde-Zimmermann D, Verdaasdonk MA, Rademakers LH, De Weger RA, Van den Tweel JG, Joling P (1997) Fibronectin distribution in human bone marrow stroma: matrix assembly and tumor cell adhesion via alpha5 beta1 integrin. Exp Cell Res 230:111–120
Acknowledgements
This work was supported by the Japan Society for the Promotion of Science (JSPS) Grants-in-Aid for Scientific Research (KAKENHI) Grant Number JP15K10427 and Japan Orthopaedics and Traumatology Foundation, Inc. Grant Number 330.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors state that they have no conflict of interest related to this study.
Ethical approval
All animal studies were performed in accordance with protocols approved by the Hokkaido University Committee on Animal Resources.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
10585_2019_9999_MOESM1_ESM.jpg
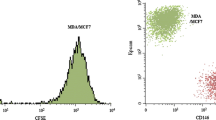
Supplementary material 1—Immunocytochemistry and gene expression analysis to confirm the expression of STn on MCF-7 breast cancer cells. (a) Immunofluorescence microscopy confirmed that ST6GalNAc I -expressing cells expressed the STn antigen on the cell surface of MCF-7 cells. (b) STn detection by flow cytometry. The presence of the STn antigen was revealed using STn mAb (black lines) and negative controls (dotted lines, secondary antibodies only). (c) Expression of the ST6GalNAc I gene was examined by quantitative PCR. Abbreviations: STn, sialyl-Tn; mAb, monoclonal antibody. RNAi, ST6GalNAc I knockdown clones from STn+ MCF-7 cells.(JPEG 781 kb)
Rights and permissions
About this article
Cite this article
Fujita, R., Hamano, H., Kameda, Y. et al. Breast cancer cells expressing cancer-associated sialyl-Tn antigen have less capacity to develop osteolytic lesions in a mouse model of skeletal colonization. Clin Exp Metastasis 36, 539–549 (2019). https://doi.org/10.1007/s10585-019-09999-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10585-019-09999-6