Abstract
We investigated the methylation status of mismatch repair gene hMLH1 in 80 primary human endometrial carcinomas (ECs) and in 30 metastatic lesions. It was correlated to the expression of hMLH1 protein, microsatellite instability (MSI) of ECs and to the well-known clinico-pathological variables of cancer. The hMLH1 promoter methylation was detected in 24 out of 64 (37.5 %) primary ECs but only in one out of 18 (5.6 %) metastatic lesions investigated. Promoter hMLH1 hypermethylation was found more often in early stage ECs and was associated with a decrease of hMLH1 protein expression immunohistochemically. An inverse relationship between hMLH1 expression and clinical stage of the disease was found (p = 0.048). Interestingly, there was a significant correlation between MSI and hMLH1 protein expression level (p = 0.042). MSI phenotype was found more often in EC metastases compared to the primary tumors (66.7 % vs 29.3 %; p = 0.039). However, neither hMLH1 promoter hypermethylation nor MSI was independent predictive factors for patient’s outcome. Using an in vitro model we showed that hMLH1 methylation is reversible. These data showed that hMLH1 methylation with a consequent protein decrease occurred early during EC tumorigenesis and may cause a MSI phenotype, which occurs relatively late. MSI may be an important mechanism supporting further the tumor progression. These findings may have importance for the specific chemosensitization of the primary tumors/metastases and can improve our understanding of endometrial carcinogenesis in humans.





Similar content being viewed by others
Abbreviations
- EC:
-
Endometrial cancer
- FIGO:
-
International Federation of Gynecology and Obstetrics
- MMR:
-
Mismatch repair
- MSP:
-
Methylation-specific PCR
- MSI:
-
Microsatellite instability
- TSG:
-
Tumor suppressor gene
- WHO:
-
World Health Organization
References
Rose DP (1996) Endometrial carcinoma. N Eng J Med 335:640–649
Amant F, Moerman P, Neven P, Timmerman D, van Limbergen E, Vegote I (2005) Endometrial cancer. Lancet 366:491–505
Sorosky JI (2008) Endometrial cancer. Obstet Gynecol 111:436–447
Bokhman JV (1983) Two pathogenetic types of endometrial carcinoma. Gynecol Oncol 15:10–17
Deligdisch L, Kase NG, Bleiweiss IJ (2000) Endometrial cancer in elderly women: a histologic and steroid receptor study. Gerontology 46:17–21
Lax SF (2004) Molecular genetic pathways in various types of endometrial carcinoma: from a phenotypical to a molecular-based classification. Virchows Arch 444:213–223
Esteller M (2002) CpG island hypermethylation and tumor suppressor genes: a booming present, a brighter future. Oncogene 21:5427–5440
Esteller M (2000) Epigenetic lesions causing genetic lesions in human cancer: promoter hypermethylation of DNA repair genes. Eur J Cancer 36:2294–2300
Herman JG, Baylin SB (2003) Gene silencing in cancer in association with promoter hypermethylation. N Eng J Med 349:2042–2054
Deng G, Chen A, Hong J, Chae HS, Kim YS (1999) Methylation of CpG in a small region of the hMLH1 promoter invariably correlates with the absence of gene expression. Cancer Res 59:2029–2033
Jones PA (2002) DNA methylation and cancer. Oncogene 21:5358–5360
Clark SJ, Melki J (2002) DNA methylation and gene silencing in cancer: which is the guilty party? Oncogene 21:5380–5387
Kulis M, Esteller M (2010) DNA methylation and cancer. Adv Genet 70:27–56
Sharma S, Kelly TK, Jones PA (2010) Epigenetics in cancer. Carcinogenesis 31:27–36
Esteller M, Levine R, Baylin SB, Ellenson LH, Herman JG (1998) MLH1 promoter hypermethylation is associated with the microsatellite instability phenotype in sporadic endometrial carcinomas. Oncogene 17:2413–2417
Esteller M, Catasus L, Matias-Guiu X, Mutter GL, Prat J, Baylin SB, Herman JG (1999) hMLH1 promoter hypermethylation is an early event in human endometrial tumorigenesis. Am J Pathol 155:1767–1772
Kanaya T, Kyo S, Maida Y, Yatabe N, Tanaka M, Nakamura M, Inoue M (2003) Frequent hypermethylation of MLH1 promoter in normal endometrium of patients with endometrial cancers. Oncogene 22:2352–2360
Simpkins SB, Bocker T, Swisher EM, Mutch DG, Gersell DJ, Kovatich AJ, Palazzo JP, Fishel R, Goodfellow PJ (1999) MLH1 promoter methylation and gene silencing is the primary cause of microsatellite instability in sporadic endometrial cancers. Hum Mol Genet 8:661–666
Strazzullo M, Cossu A, Baldinu P, Colombino M, Satta MP, Tanda F, De Bonis ML, Cerase A, D’Urso M, D’Esposito M, Palmieri G (2003) High-resolution methylation analysis of the hMLH1 promoter in sporadic endometrial and colorectal carcinomas. Cancer 98:1540–1546
Yang HJ, Liu VW, Wang Y, Tsang PC, Ngan HY (2006) Differential DNA methylation profiles in gynecological cancers and correlation with clinico-pathological data. BMC Cancer 6:212
Zighelboim I, Goodfellow PJ, Gao F, Gibb RK, Powell MA, Rader JS, Mutch DG (2007) Microsatellite instability and epigenetic inactivation of MLH1 and outcome of patients with endometrial carcinomas of the endometrioid type. J Clin Oncol 25:2042–2048
Guida M, Sanguedolce F, Bufo P, Di Spiezio Sardo A, Bifulco G, Nappi C, Pannone G (2009) Aberrant DNA hypermethylation of hMLH-1 and CKDN2A/p16 genes in benign, premalignant and malignant endometrial lesions. Eur J Gynaecol Oncol 30:267–270
Muraki Y, Banno K, Yanokura M, Kobayashi Y, Kawaguchi M, Nomura H, Hirasawa A, Susumu N, Aoki D (2009) Epigenetic DNA hypermethylation: clinical applications in endometrial cancer (Review). Oncol Rep 22:967–972
Di Domenico M, Santoro A, Ricciardi C, Iaccarino M, Iaccarino S, Freda M, Feola A, Sanguedolce F, Losito S, Pasquali D, Di Spiezio Sardo A, Bifulco G, Nappi C, Bufo P, Guida M, De Rosa G, Abbruzzese A, Caraglia M, Pannone G (2011) Epigenetic fingerprint in endometrial carcinogenesis: the hypothesis of a uterine field cancerization. Cancer Biol Ther 12:447–457
Thomas DC, Umar A, Kunkel TA (1996) Microsatellite instability and mismatch repair defects in cancer. Mutat Res 350:201–205
Gurin CC, Federici MG, Kang L, Boyd J (1999) Causes and consequences of microsatellite instability in endometrial carcinoma. Cancer Res 59:462–466
Furlan D, Carnevali I, Marcomini B, Cerutti R, Dainese E, Capella C, Riva C (2006) The high frequency of de novo promoter methylation in synchronous primary endometrial and ovarian carcinomas. Clin Cancer Res 12:3329–3336
Pijnenborg JM, Dam-de Veen GC, de Haan J, van Engeland M, Groothuis PG (2004) Defective mismatch repair and the development of recurrent endometrial carcinoma. Gynecol Oncol 94:550–559
Hirasawa A, Aoki D, Inoue J, Imoto I, Susumu N, Sugano K, Nozawa S, Inazawa J (2003) Unfavorable prognostic factors associated with high frequency of microsatellite instability and comparative genomic hybridization analysis in endometrial cancer. Clin Cancer Res 9:5675–5682
An HJ, Kim KI, Kim JY, Shim JY, Kang H, Kim TH, Kim JK, Jeong JK, Lee SY, Kim SJ (2007) Microsatellite instability in endometrioid type endometrial adenocarcinoma is associated with poor prognostic indicators. Am J Surg Pathol 31:846–853
Black D, Soslow RA, Levine DA, Tornos C, Chen SC, Hummer AJ, Bogomolniy F, Olvera N, Barakat RR, Boyd J (2006) Clinicopathologic significance of defective DNA mismatch repair in endometrial carcinoma. J Clin Oncol 24:1745–1753
Caduff RF, Johnston CM, Svoboda-Newman SM, Poy EL, Merajver SD, Frank TS (1996) Clinical and pathological significance of microsatellite instability in sporadic endometrial carcinoma. Am J Pathol 148:1671–1678
Katabuchi H, van Rees B, Lambers AR, Ronnett BM, Blazes MS, Leach FS, Cho KR, Hedrick L (1995) Mutations in DNA mismatch repair genes are not responsible for microsatellite instability in most sporadic endometrial carcinomas. Cancer Res 55:5556–5560
Maxwell GL, Risinger JI, Alvarez AA, Barrett JC, Berchuck A (2001) Favorable survival associated with microsatellite instability in endometrioid endometrial cancers. Obstet Gynecol 97:417–422
Peiro G, Diebold J, Lohse P, Ruebsamen H, Lohse P, Baretton GB, Lohrs U (2002) Microsatellite instability, loss of heterozygosity, and loss of hMLH1 and hMSH2 protein expression in endometrial carcinoma. Hum Pathol 33:347–354
Scully RE, Bonfiglio TA, Kurman RJ, Silverberg SG, Wilkinson EJ (1994) Histological typing of female genital tract tumours, 2nd edn. Springer, Berlin
Pecorelli S (2009) Revised FIGO staging for carcinoma of the vulva, cervix, and endometrium. Int J Gynaecol Obstet 105:103–104
Semczuk A, Boltze C, Marzec B, Szczygielska A, Roessner A, Schneider-Stock R (2003) p16 INK4A alterations are accompanied by aberrant protein immunostaining in endometrial carcinomas. J Cancer Res Clin Oncol 129:589–596
Ignatov A, Bischoff J, Ignatov T, Schwarzenau C, Krebs T, Kuester D, Costa SD, Roessner A, Semczuk A, Schneider-Stock R (2010) APC promoter hypermethylation is an early event in endometrial tumorigenesis. Cancer Sci 101:321–327
Ignatov A, Bischoff J, Schwarzenau C, Krebs T, Kuester D, Herrmann K, Costa SD, Roessner A, Semczuk A, Schneider-Stock R (2008) P16 alterations increase the metastatic potential of endometrial carcinoma. Gynecol Oncol 111:365–371
Boland CR, Thibodeau SN, Hamilton SR, Sidransky D, Eshleman JR, Burt RW, Meltzer SJ, Rodriguez-Bigas MA, Fodde R, Ranzani GN, Srivastava S (1998) A National Cancer Institute Workshop on Microsatellite Instability for cancer detection and familial predisposition: development of international criteria for the determination of microsatellite instability in colorectal cancer. Cancer Res 58:5248–5257
Shibata D, Peinado MA, Ionov Y, Malkhosyan S, Perucho M (1994) Genomic instability in repeated sequences is an early somatic event in colorectal tumorigenesis that persists after transformation. Nat Genet 6:273–281
Ignatov A, Ignatov T, Roessner A, Costa SD, Kalinski T (2010) Role of GPR30 in the mechanisms of tamoxifen resistance in breast cancer MCF-7 cells. Breast Cancer Res Treat 123:87–96
Kondo E, Furukawa T, Yoshinaga K, Kijima H, Semba S, Yatsuoka T, Yokoyama T, Fukushige S, Horii A (2000) Not hMLH2 but hMLH1 is frequently silenced by hypermethylation in endometrial cancer but rarely silenced in pancreatic cancer with microsatellite instability. Int J Oncol 17:535–541
Salvesen H, McDonald N, Ryan A, Iversen OE, Jacobs IJ, Akslen LA, Das S (2000) Methylation of hMLH1 in a population-based series of endometrial carcinomas. Clin Cancer Res 6:3607–3613
Banno K, Yanokura M, Susumu N, Kawaguchi M, Hirao N, Hirasawa A, Tsukazaki K, Aoki D (2006) Relationship of the aberrant DNA hypermethylation of cancer-related genes with carcinogenesis of endometrial cancer. Oncol Rep 16:1189–1196
Kang S, Kim JW, Kang GH, Lee S, Park NH, Song YS, Park SY, Kang SB, Lee HP (2006) Comparison of DNA hypermethylation patterns in different types of uterine cancer: cervical squamous cell carcinoma, cervical adenocarcinoma and endometrial adenocarcinoma. Int J Cancer 118:2168–2171
Xiong Y, Dowdy SC, Eberhardt NL, Podratz KC, Jiang SW (2006) hMLH1 promoter methylation and silencing in primary endometrial cancers are associated with specific alterations in MBDs occupancy and histone modifications. Gynecol Oncol 103:321–328
Kim MS, Lee J, Sidransky D (2010) DNA methylation markers in colorectal cancer. Cancer Metastasis Rev 29:181–206
Kawaguchi M, Banno K, Yanokura M, Kobayashi Y, Kishimi A, Ogawa S, Kisu I, Nomura H, Hirasawa A, Susumu N, Aoki D (2009) Analysis of candidate target genes for mononucleotide repeat mutation in microsatellite instability-high (MSI-H) endometrial cancer. Int J Oncol 35:977–982
Herman JG, Umar A, Polyak K, Graff JR, Ahuja N, Issa JP, Markowitz S, Willson JK, Hamilton SR, Kinzler KW, Kane MF, Kolodner RD, Vogelstein B, Kunkel TA, Baylin SB (1998) Incidence and functional consequences of hMLH1 promoter hypermethylation in colorectal carcinoma. Proc Natl Acad Sci USA 95:6870–6875
Fleisher AS, Esteller M, Tamura G, Rashid A, Stine OC, Yin J, Zou TT, Abraham JM, Kong D, Nishizuka S, James SP, Wilson KT, Herman JG, Meltzer SJ (2001) Hypermethylation of the hMLH1 gene promoter is associated with microsatellite instability in early human gastric neoplasia. Oncogene 20:329–335
Loeb LA (1994) Microsatellite instability: marker of a mutator phenotype in cancer. Cancer Res 54:5059–5063
Leung SY, Yuen ST, Chung LP, Chu KM, Chan AS, Ho JC (1999) hMLH1 promoter methylation and lack of hMLH1 expression in sporadic gastric carcinomas with high-frequency microsatellite instability. Cancer Res 59:159–164
Fleisher AS, Esteller M, Wang S, Tamura G, Suzuki H, Yin J, Zou TT, Abraham JM, Kong D, Smolinski KN, Shi YQ, Rhyu MG, Powell SM, James SP, Wilson KT, Herman JG, Meltzer SJ (1999) Hypermethylation of the hMLH1 gene promoter in human gastric cancers with microsatellite instability. Cancer Res 59:1090–1095
Fink D, Aebi S, Howell SB (1984) The role of DNA mismatch repair in drug resistance. Clin Cancer Res 4:1–6
Steele N, Finn P, Brown R, Plumb JA (2009) Combined inhibition of DNA methylation and histone acetylation enhances gene re-expression and drug sensitivity in vivo. Br J Cancer 100:758–763
Jo WS, Carethers JM (2006) Chemotherapeutic implications in microsatellite unstable colorectal cancer. Cancer Biomark 2:51–60
Liang J-T, Huang K-C, Lai H-S, Lee P-H, Cheng Y-M, Hsu Y-C, Cheng A-L, Hsu C-H, Yeh K-H, Wang S-M, Tang C, Chang K-J (2002) High-frequency microsatellite instability predicts better chemosensitivity to high-dose 5-fluorouracil plus leucovorin chemotherapy for stage IV sporadic colorectal cancer after palliative bowel resection. Int J Cancer 101:519–525
Magrini R, Bhonde MR, Hanski ML, Notter M, Scherubl H, Boland CR, Zeitz M, Hanski C (2002) Cellular effects of CPT-11 on colon carcinoma cells: dependence on p53 and hMLH1 status. Int J Cancer 101:23–31
Fallik D, Borrini F, Boige V, Viguier J, Jacob S, Miquel C, Sabourin JC, Ducreux M, Praz F (2003) Microsatellite instability is a predictive factor of the tumor response to irinotecan in patients with advanced colorectal cancer. Cancer Res 63:5738–5744
Basil JB, Goodfellow PJ, Rader JS, Mutch DG, Herzog TJ (2000) Clinical significance of microsatellite instability in endometrial carcinoma. Cancer 89:1758–1764
Jeczen R, Skomra D, Cybulski M, Schneider-Stock R, Szewczuk W, Roessner A, Rechberger T, Semczuk A (2007) P53/MDM2 overexpression in metastatic endometrial cancer: correlation with clinicopathological features and patient outcome. Clin Exp Metastasis 24:503–511
Szewczuk W, Skomra D, Cybulski M, Prządka-Rabaniuk D, Filip A, Jozwik M, Olcha P, Roessner A, Semczuk A (2009) Allelic loss at TP53 in metastatic human endometrial carcinomas. Clin Exp Metastasis 26:789–796
Semczuk A, Schneider-Stock R, Szewczuk W (2010) Prevalence of allelic loss at TP53 in endometrial carcinomas. (Review). Oncology 78:220–228
Acknowledgments
We thank Dr. E. Erbstößer, Dr. K. Hellwig and Dr. M. Löttge for providing us paraffin-embedded specimens. We are grateful to the whole team of the laboratory of immunohistochemistry the staff of the Department of Pathology, Otto-von-Guericke University, Magdeburg, Germany, and the Department of Pathology, Lublin Medical University, Lublin, Poland, for the excellent technical assistance. This work was supported by NBL3-2 by the BMBF (Förderkennzeichen 01ZZ0407).
Conflict of interest
The authors declare that there are no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
J. Bischoff and A. Ignatov contributed equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
10585_2012_9478_MOESM1_ESM.ppt
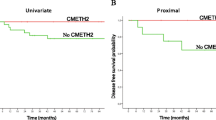
Fig. 1: Patient survival dependent on hMLH1 methylated status. A) Overall survival in all cohort, B) Progression-free survival in all cohort, C) Overall survival in early stage endometrial carcinoma (FIGO I/II), D) Progression-free survival in early stage endometrial carcinoma (FIGO I/II). Broken line represents the methylated cases, unbroken line represents the unmethylated cases. (PPT 141 kb)
Rights and permissions
About this article
Cite this article
Bischoff, J., Ignatov, A., Semczuk, A. et al. hMLH1 promoter hypermethylation and MSI status in human endometrial carcinomas with and without metastases. Clin Exp Metastasis 29, 889–900 (2012). https://doi.org/10.1007/s10585-012-9478-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10585-012-9478-0