Abstract
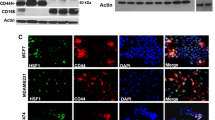
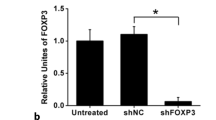
Cancer cells metastasize by entering the lymphatic system. Regional lymph-node dissemination is the first detectable step in the metastasis of oral squamous cell carcinoma (SCC) and is highly correlated to the prognosis of the disease. Cold shock domain protein A (CSDA) is a DNA-binding protein that represses angiogenesis and lymphangiogenesis by directly binding to hypoxia response element (HRE) and serum response element (SRE). In our study we used the cell line NR-S1M, a mouse SCC model with a high rate of lymph-node metastasis. Into these cells we transfected the expression-plasmid coding for full-length mouse CSDA. Of importance, we showed that overexpression of CSDA significantly inhibits the production of VEGF-A and VEGF-C in NR-S1M cells. The overexpression of CSDA in NR-S1M cells inhibited tumor growth, inhibited regional lymph-node metastasis, and reduced the density of blood vessels and lymphatic vessels in the primary tumors in vivo. Our results support the hypothesis that VEGF-A and VEGF-C are crucial regulators of angiogenesis and lymphangiogenesis in NR-S1M cells. Therefore, they are promising targets for CSDA overexpression gene therapy to inhibit tumor growth and lymph-node metastasis in SCC.




Similar content being viewed by others
References
Rennie J, Rusting R (1996) Making headway against cancer. Sci Am 275:56–59
Fidler IJ (2001) Molecular biology of cancer: invasion and metastasis. In: DeVita VT, Hellman S Jr, Rosenburg SA (eds) Cancer Principles and Practice of Oncology. Lippincott Raven, Philadelphia, pp 135–153
Karpanen T, Alitalo K (2001) Lymphatic vessels as targets of tumor therapy? J Exp Med 194:37–42
Fisher B, Bauer M, Wickerham DL et al (1983) Relation of number of positive axillary nodes to the prognosis of patients with primary breast cancer. An NSABP update. Cancer 52:1551–1557
Pepper MS (2001) Lymphangiogenesis and tumor metastasis: myth or reality? Clin Cancer Res 7:462–468
Wells KE, Rapaport DP, Cruse CW et al (1997) Sentinel lymph node biopsy in melanoma of the head and neck. Plast Reconstr Surg 100:591–594
Albertini JJ, Lyman GH, Cox C et al (1996) Lymphatic mapping and sentinel node biopsy in the patient with breast cancer. J Am Med Assoc 276:1818–1822
Albertini JJ, Cruse CW, Rapaport D et al (1996) Intraoperative radio-lympho-scintigraphy improves sentinel lymph node identification for patients with melanoma. Ann Surg 223:217–224
Mandriota SJ, Jussila L, Jeltsch M et al (2001) Vascular endothelial growth factor-C-mediated lymphangiogenesis promotes tumour metastasis. EMBO J 20:672–682
Skobe M, Hawighorst T, Jackson DG et al (2001) Induction of tumor lymphangiogenesis by VEGF-C promotes breast cancer metastasis. Nat Med 7:192–198
Stacker SA, Caesar C, Baldwin ME et al (2001) VEGF-D promotes the metastatic spread of tumor cells via the lymphatics. Nat Med 7:186–191
Karpanen T, Egeblad M, Karkkainen MJ et al (2001) Vascular endothelial growth factor C promotes tumor lymphangiogenesis and intralymphatic tumor growth. Cancer Res 61:1786–1790
Shannon MF, Coles LS, Vadas MA et al (1997) Signals for activation of the GM-CSF promoter and enhancer in T cells. Crit Rev Immunol 17:301–323
Shannon MF, Coles LS, Attema J et al (2001) The role of architectural transcription factors in cytokine gene transcription. J Leukoc Biol 69:21–32
Coles LS, Diamond P, Lambrusco L et al (2002) A novel mechanism of repression of the vascular endothelial growth factor promoter, by single strand DNA binding cold shock domain (Y-box) proteins in normoxic fibroblasts. Nucleic Acids Res 30:4845–4854
Saito Y, Nakagami H, Kurooka M et al (2008) Cold shock domain protein A represses angiogenesis and lymphangiogenesis via inhibition of serum response element. Oncogene 27:1821–1833
Usui S, Urano M, Koike S et al (1976) Effect of PS-K, a protein polysaccharide, on pulmonary metastases of a C3H mouse squamous cell carcinoma. J Natl Cancer Inst 56:185–187
Clauss M, Gerlach M, Gerlach H et al (1990) Vascular permeability factor: a tumor-derived polypeptide that induces endothelial cell and monocyte procoagulant activity, and promotes monocyte migration. J Exp Med 172:1535–1545
Krishnan J, Kirkin V, Steffen A et al (2003) Differential in vivo and in vitro expression of vascular endothelial growth factor (VEGF)-C and VEGF-D in tumors and its relationship to lymphatic metastasis in immunocompetent rats. Cancer Res 63:713–722
Gleadle JM, Ratcliffe PJ (1997) Induction of hypoxia-inducible factor-1, erythropoietin, vascular endothelial growth factor, and glucose transporter-1 by hypoxia: evidence against a regulatory role for Src kinase. Blood 89:503–509
Forsythe JA, Jiang BH, Iyer NV et al (1996) Activation of vascular endothelial growth factor gene transcription by hypoxia-inducible factor 1. Mol Cell Biol 16:4604–4613
Hong YK, Lange-Asschenfeldt B, Velasco P et al (2004) VEGF-A promotes tissue repair-associated lymphatic vessel formation via VEGFR-2 and the alpha1beta1 and alpha2beta1 integrins. FASEB J 18:1111–1113
Saaristo A, Veikkola T, Enholm B et al (2002) Adenoviral VEGF-C overexpression induces blood vessel enlargement, tortuosity, and leakiness but no sprouting angiogenesis in the skin or mucous membranes. FASEB J 16:1041–1049
Kimura H, Konishi K, Nukui T et al (2001) Prognostic significance of expression of thymidine phosphorylase and vascular endothelial growth factor in human gastric carcinoma. J Surg Oncol 7:31–36
Hirakawa S, Kodama S, Kunstfeld R et al (2005) VEGF-A induces tumor and sentinel lymph node lymphangiogenesis and promotes lymphatic metastasis. J Exp Med 201:1089–1099
Skobe M, Detmar M (2000) Structure, function, and molecular control of the skin lymphatic system. J Investig Dermatol Symp Proc 5:14–19
Yonemura Y, Endo Y, Fujita H et al (1999) Role of vascular endothelial growth factor C expression in the development of lymph node metastasis in gastric cancer. Clin Cancer Res 5:1823–1829
Akagi K, Ikeda Y, Miyazaki M et al (2000) Vascular endothelial growth factor-C (VEGF-C) expression in human colorectal cancer tissues. Br J Cancer 83:887–891
Niki T, Iba S, Tokunou M et al (2000) Expression of vascular endothelial growth factors A, B, C, and D and their relationships to lymph node status in lung adenocarcinoma. Clin Cancer Res 6:2431–2439
Sugiura T, Inoue Y, Matsuki R et al (2009) VEGF-C and VEGF-D expression is correlated with lymphatic vessel density and lymph node metastasis in oral squamous cell carcinoma: implications for use as a prognostic marker. Int J Oncol 34:673–680
Isaka N, Padera TP, Hagendoorn J et al (2004) Peritumor lymphatics induced by vascular endothelial growth factor-C exhibit abnormal function. Cancer Res 64:4400–4404
Chilov D, Kukk E, Taira S et al (1997) Genomic organization of human and mouse genes for vascular endothelial growth factor C. J Biol Chem 272:25176–25183
Okada K, Osaki M, Araki K et al (2005) Expression of hypoxia-inducible factor (HIF-1alpha), VEGF-C and VEGF-D in non-invasive and invasive breast ductal carcinomas. Anticancer Res 25:3003–3009
Nilsson I, Shibuya M, Wennström S (2004) Differential activation of vascular genes by hypoxia in primary endothelial cells. Exp Cell Res 299:476–485
Joukov V, Sorsa T, Kumar V et al (1997) Proteolytic processing regulates receptor specificity and activity of VEGF-C. EMBO J 16:3898–3911
Fujii T, Otsuki T, Moriya T et al (2002) Effect of hypoxia on human seminoma cells. Int J Oncol 20:955–962
Katsuta M, Miyashita M, Makino H et al (2005) Correlation of hypoxia inducible factor-1alpha with lymphatic metastasis via vascular endothelial growth factor-C in human esophageal cancer. Exp Mol Pathol 78:123–130
Beasley NJ, Prevo R, Banerji S et al (2002) Intratumoral lymphangiogenesis and lymph node metastasis in head and neck cancer. Cancer Res 62:1315–1326
Sanna-Mari M, Marjaana L, Reidar G et al (2003) Intratumoral lymphatics are essential for the metastatic spread and prognosis in squamous sell carcinomas of the head and neck region. Cancer Res 63:1920
Kishimoto K, Sasaki A, Yoshihama Y et al (2003) Expression of vascular endothelial growth factor-C predicts regional lymph node metastasis in early oral squamous cell carcinoma. Oral Oncol 39:391–396
Acknowledgments
The Ministry of Education, Culture, Sports, Science and Technology of Japan supported this work with a Grant-in-Aid for the High-Tech Research Center Project.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Matsumoto, G., Yajima, N., Saito, H. et al. Cold shock domain protein A (CSDA) overexpression inhibits tumor growth and lymph node metastasis in a mouse model of squamous cell carcinoma. Clin Exp Metastasis 27, 539–547 (2010). https://doi.org/10.1007/s10585-010-9343-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10585-010-9343-y