Abstract
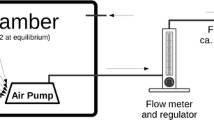
The pulse decay discrimination (PDD) liquid scintillation technique has been applied to optimise radon counting by the Pico-Rad method. A dermination limit (with 10% relative error) of 4.8 Bqm−3 for indoor radon measurement has been achieved for optimal PDD setting with a radon elution cocktail containing 20% (v/v) of Ultima Gold AB in Instafluor. From a practical point of view this procedure allows a shortening of the counting time to 1 hour after 48 hours exposure to detectors. This method has been applied to indoor radon determinations in 626 places (municipal offices and private dwellings) in the Lódz region. These measureents resulted in an average concentration of 21.4 Bqm−3 and a median value of 15.1 Bqm−3. Analysis of the data indicates that most indoor radon comes from the underlying soil, which contains relatively little226Ra (10–20 Bqkg−1).
Similar content being viewed by others
References
J. H. Lubin and J. D. Boice, J. Nat. Cancer Inst.89 (1997) 49.
F. Schönhofer, K. Pock and H. Friedman: J. Radioanal. Nucl. Chem., Art.193 (1995) 337.
H. Bem, T. Domanski, Y. Y. Bakir and S. Al Zenki:in Proc. Int. Conf. IRPA 9 Vienna, 1996, Vol. 2 (1996), p. 101.
J. Maringer et al.:in Proc. IRPA Symp. Prague, 1997, (Ed. J. Sabol) Prague, 1998, p. 150.
L. A. Currie: Anal. Chem.40 (1968) 586
L. Salonen: Sci. Total Environ.130/131, (1993) 23.
J. D. Spalding and J. E. Noakes:in Liquid Scintillation Spectrometry, Tucson, 1992, (Eds. J. E. Noakes, F. Schönhofer and M. A. Polach), Radiocarbon, 1993, p. 373.
S. Möbius, P. Kamolchote, T. L. Ramamonjisoa and M. Yang:in Liquid Scintillation Spectrometry, Tucson, 1992 (Eds. J. E. Noakes, F. Schönhofer and M. A. Polach), Radiocarbon, 1993, p. 413.
H. Bem, Y. Y. Bakir and F. Bou-Rabee: J. Radioanal. Nucl. Chem., Lett.186 (1994) 119.
W. J. McDowell:in Proc.Liquid Scintillation Spectrometry, Glasgow, 1994, (Eds. G. T. Cook, D. D. Harkness, A. B. Mackenzie, B. F. Miller and E. M. Scott), Radiocarbon, 1996, p. 327.
T. Oikari, H. Kojola, J. Nurmi and L. Kaihola, Appl. Radiat. Isot.38 (1987) 875.
K. Mamont-Ciesla et al.:in Proc. International Conference onTechnologically Enhanced Natural Radiation, Szczyrk, Poland, 1996, p. 333.
J. Vaupotic, M. Szymula, J. Solecki, S. Chibowski and I. Kobal: Health Phys.64 (1993) 422.
H. Bem and P. Wieczorkowski, unpublished results.
International Atomic Energy Agency:Basic Safety Standards for Radiation Protection. Safety Series No. 115 1, Viena, 1994, p. 100.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Bem, H., Ostrowska, M. & Bem, E.M. Application of pulse decay discrimination liquid scintillation counting for indoor radon measurement. Czech J Phys 49 (Suppl 1), 97–102 (1999). https://doi.org/10.1007/s10582-999-0012-9
Issue Date:
DOI: https://doi.org/10.1007/s10582-999-0012-9