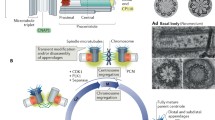
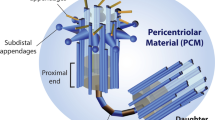
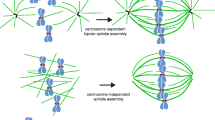
Abstract
The maintenance of genome stability is critical for proper cell function, and loss of this stability contributes to many human diseases and developmental disorders. Therefore, cells have evolved partially redundant mechanisms to monitor and protect the genome. One subcellular organelle implicated in the maintenance of genome stability is the centrosome, best known as the primary microtubule organizing center of most animal cells. Centrosomes serve many different roles throughout the cell cycle, and many of those roles, including mitotic spindle assembly, nucleation of the interphase microtubule array, DNA damage response, and efficient cell cycle progression, have been proposed to help maintain genome stability. As a result, the centrosome is itself a highly regulated entity. Here, we review evidence concerning the significance of the centrosome in promoting genome integrity. Recent advances permitting acute and persistent centrosome removal suggest we still have much to learn regarding the specific function and actual importance of centrosomes in different contexts, as well as how cells may compensate for centrosome dysfunction to maintain the integrity of the genome. Although many animal cells survive and proliferate in the absence of centrosomes, they do so aberrantly. Based on these and other studies, we conclude that centrosomes serve as critical, multifunctional organelles that promote genome stability.




Similar content being viewed by others
Abbreviations
- CIN:
-
Chromosomal instability
- MT:
-
Microtubule
- MTOC:
-
Microtubule organizing center
- aMTOC:
-
Acentriolar microtubule organizing center
- PCM:
-
Pericentriolar material
- RNAi:
-
RNA interference
- NEB:
-
Nuclear envelope breakdown
- SAC:
-
Spindle assembly checkpoint
- NSC:
-
Neural stem cell
- mGSC:
-
Male germline stem cell
References
Abraham RT (2001) Cell cycle checkpoint signaling through the ATM and ATR kinases. Genes Dev 15:2177–2196
Arquint C, Gabryjonczyk AM, Nigg EA (2014) Centrosomes as signalling centres. Philos Trans R Soc Lond B Biol Sci 369:20130464
Basto R, Lau J, Vinogradova T, Gardiol A, Woods CG, Khodjakov A, Raff JW (2006) Flies without centrioles. Cell 125:1375–1386
Baumbach J, Novak ZA, Raff JW, Wainman A (2015) Dissecting the function and assembly of acentriolar microtubule organizing centers in Drosophila cells in vivo. PLoS Genet 11:e1005261
Bazzi H, Anderson KV (2014) Acentriolar mitosis activates a p53-dependent apoptosis pathway in the mouse embryo. Proc Natl Acad Sci U S A 111:E1491–E1500
Blachon S, Gopalakrishnan J, Omori Y, Polyanovsky A, Church A, Nicastro D, Malicki J, Avidor-Reiss T (2008) Drosophila asterless and vertebrate Cep152 are orthologs essential for centriole duplication. Genetics 180:2081–2094
Bolgioni AF, Ganem NJ (2015) The interplay between centrosomes and the hippo tumor suppressor pathway. Chromosome Res doi:10.1007/s10577-015-9502-8
Bonaccorsi S, Giansanti MG, Gatti M (1998) Spindle self-organization and cytokinesis during male meiosis in asterless mutants of Drosophila melanogaster. J Cell Biol 142:751–761
Borel F, Lohez OD, Lacroix FB, Margolis RL (2002) Multiple centrosomes arise from tetraploidy checkpoint failure and mitotic centrosome clusters in p53 and RB pocket protein-compromised cells. Proc Natl Acad Sci U S A 99:9819–9824
Brito DA, Rieder CL (2006) Mitotic checkpoint slippage in humans occurs via cyclin B destruction in the presence of an active checkpoint. Curr Biol 16:1194–1200
Brito DA, Yang Z, Rieder CL (2008) Microtubules do not promote mitotic slippage when the spindle assembly checkpoint cannot be satisfied. J Cell Biol 182:623–629
Buffin E, Emre D, Karess RE (2007) Flies without a spindle checkpoint. Nat Cell Biol 9:565–572
Canman JC, Cameron LA, Maddox PS, Straight A, Tirnauer JS, Mitchison TJ, Fang G, Kapoor TM, Salmon ED (2003) Determining the position of the cell division plane. Nature 424:1074–1078
Castellanos E, Dominguez P, Gonzalez C (2008) Centrosome dysfunction in Drosophila neural stem cells causes tumors that are not due to genome instability. Curr Biol 18:1209–1214
Cimini D, Howell B, Maddox P, Khodjakov A, Degrassi F, Salmon ED (2001) Merotelic kinetochore orientation is a major mechanism of aneuploidy in mitotic mammalian tissue cells. J Cell Biol 153:517–527
Cosenza MR, Krämer A (2015) Centrosome amplification, chromosomal instability and cancer: mechanistic, clinical and therapeutic issues. Chromosome Res. In press
Crasta K, Ganem NJ, Dagher R, Lantermann AB, Ivanova EV, Pan Y, Nezi L, Protopopov A, Chowdhury D, Pellman D (2012) DNA breaks and chromosome pulverization from errors in mitosis. Nature 482:53–58
Dekanty A, Barrio L, Muzzopappa M, Auer H, Milan M (2012) Aneuploidy-induced delaminating cells drive tumorigenesis in Drosophila epithelia. Proc Natl Acad Sci U S A 109:20549–20554
Doxsey S, McCollum D, Theurkauf W (2005) Centrosomes in cellular regulation. Annu Rev Cell Dev Biol 21:411–434
Feldman JL, Priess JR (2012) A role for the centrosome and PAR-3 in the hand-off of MTOC function during epithelial polarization. Curr Biol 22:575–582
Foley EA, Kapoor TM (2013) Microtubule attachment and spindle assembly checkpoint signalling at the kinetochore. Nat Rev Mol Cell Biol 14:25–37
Fox DT, Gall JG, Spradling AC (2010) Error-prone polyploid mitosis during normal Drosophila development. Genes Dev 24:2294–2302
Fukasawa K (2007) Oncogenes and tumour suppressors take on centrosomes. Nat Rev Cancer 7:911–924
Goshima G, Mayer M, Zhang N, Stuurman N, Vale RD (2008) Augmin: a protein complex required for centrosome-independent microtubule generation within the spindle. J Cell Biol 181:421–429
Green RA, Paluch E, Oegema K (2012) Cytokinesis in animal cells. Annu Rev Cell Dev Biol 28:29–58
Hamill DR, Severson AF, Carter JC, Bowerman B (2002) Centrosome maturation and mitotic spindle assembly in C. elegans require SPD-5, a protein with multiple coiled-coil domains. Dev Cell 3:673–684
Harris TJ, Peifer M (2007) aPKC controls microtubule organization to balance adherens junction symmetry and planar polarity during development. Dev Cell 12:727–738
Hashimoto T (2013) A ring for all: gamma-tubulin-containing nucleation complexes in acentrosomal plant microtubule arrays. Curr Opin Plant Biol 16:698–703
Hayward D, Metz J, Pellacani C, Wakefield JG (2014) Synergy between multiple microtubule-generating pathways confers robustness to centrosome-driven mitotic spindle formation. Dev Cell 28:81–93
Heald R, Tournebize R, Blank T, Sandaltzopoulos R, Becker P, Hyman A, Karsenti E (1996) Self-organization of microtubules into bipolar spindles around artificial chromosomes in Xenopus egg extracts. Nature 382:420–425
Heinrichs A (2007) A centrosome-integrity checkpoint. Nat Rev Mol Cell Biol 8:98
Hinchcliffe EH, Miller FJ, Cham M, Khodjakov A, Sluder G (2001) Requirement of a centrosomal activity for cell cycle progression through G1 into S phase. Science 291:1547–1550
Holland AJ, Cleveland DW (2009) Boveri revisited: chromosomal instability, aneuploidy and tumorigenesis. Nat Rev Mol Cell Biol 10:478–487
Hornick JE, Mader CC, Tribble EK, Bagne CC, Vaughan KT, Shaw SL, Hinchcliffe EH (2011) Amphiastral mitotic spindle assembly in vertebrate cells lacking centrosomes. Curr Biol 21:598–605
Hut HM, Lemstra W, Blaauw EH, Van Cappellen GW, Kampinga HH, Sibon OC (2003) Centrosomes split in the presence of impaired DNA integrity during mitosis. Mol Biol Cell 14:1993–2004
Inanc B, Dodson H, Morrison CG (2010) A centrosome-autonomous signal that involves centriole disengagement permits centrosome duplication in G2 phase after DNA damage. Mol Biol Cell 21:3866–3877
Insolera R, Bazzi H, Shao W, Anderson KV, Shi SH (2014) Cortical neurogenesis in the absence of centrioles. Nat Neurosci 17:1528–1535
Janssen A, van der Burg M, Szuhai K, Kops GJ, Medema RH (2011) Chromosome segregation errors as a cause of DNA damage and structural chromosome aberrations. Science 333:1895–1898
Januschke J, Llamazares S, Reina J, Gonzalez C (2011) Drosophila neuroblasts retain the daughter centrosome. Nat Commun 2:243
Januschke J, Reina J, Llamazares S, Bertran T, Rossi F, Roig J, Gonzalez C (2013) Centrobin controls mother-daughter centriole asymmetry in Drosophila neuroblasts. Nat Cell Biol 15:241–248
Kapoor TM, Mayer TU, Coughlin ML, Mitchison TJ (2000) Probing spindle assembly mechanisms with monastrol, a small molecule inhibitor of the mitotic kinesin, Eg5. J Cell Biol 150:975–988
Kaseda K, McAinsh AD, Cross RA (2012) Dual pathway spindle assembly increases both the speed and the fidelity of mitosis. Biol Open 1:12–18
Kastan MB, Onyekwere O, Sidransky D, Vogelstein B, Craig RW (1991) Participation of p53 protein in the cellular response to DNA damage. Cancer Res 51:6304–6311
Khodjakov A, Rieder CL (1999) The sudden recruitment of gamma-tubulin to the centrosome at the onset of mitosis and its dynamic exchange throughout the cell cycle, do not require microtubules. J Cell Biol 146:585–596
Khodjakov A, Rieder CL (2001) Centrosomes enhance the fidelity of cytokinesis in vertebrates and are required for cell cycle progression. J Cell Biol 153:237–242
Khodjakov A, Cole RW, Oakley BR, Rieder CL (2000) Centrosome-independent mitotic spindle formation in vertebrates. Curr Biol 10:59–67
Lambrus BG, Uetake Y, Clutario KM, Daggubati V, Snyder M, Sluder G, Holland AJ (2015) p53 protects against genome instability following centriole duplication failure. J Cell Biol 210:63–77
Lavia P (2015) The GTPase RAN regulates multiple steps of the centrosome life cycle. Chromosome Res. in press
Lecland N, Debec A, Delmas A, Moutinho-Pereira S, Malmanche N, Bouissou A, Dupre C, Jourdan A, Raynaud-Messina B, Maiato H et al (2013) Establishment and mitotic characterization of new Drosophila acentriolar cell lines from DSas-4 mutant. Biol Open 2:314–323
Lerit DA, Rusan NM (2013) PLP inhibits the activity of interphase centrosomes to ensure their proper segregation in stem cells. J Cell Biol 202:1013–1022
Lerit DA, Jordan HA, Poulton JS, Fagerstrom CJ, Galletta BJ, Peifer M, Rusan NM (2015) Interphase centrosome organization by the PLP-Cnn scaffold is required for centrosome function. J Cell Biol 210:79–97
Li K, Xu EY, Cecil JK, Turner FR, Megraw TL, Kaufman TC (1998) Drosophila centrosomin protein is required for male meiosis and assembly of the flagellar axoneme. J Cell Biol 141:455–467
Loffler H, Fechter A, Liu FY, Poppelreuther S, Kramer A (2013) DNA damage-induced centrosome amplification occurs via excessive formation of centriolar satellites. Oncogene 32:2963–2972
Meads T, Schroer TA (1995) Polarity and nucleation of microtubules in polarized epithelial cells. Cell Motil Cytoskeleton 32:273–288
Megraw TL, Li K, Kao LR, Kaufman TC (1999) The centrosomin protein is required for centrosome assembly and function during cleavage in Drosophila. Development 126:2829–2839
Megraw TL, Kao LR, Kaufman TC (2001) Zygotic development without functional mitotic centrosomes. Curr Biol 11:116–120
Mikule K, Delaval B, Kaldis P, Jurcyzk A, Hergert P, Doxsey S (2007) Loss of centrosome integrity induces p38-p53-p21-dependent G1-S arrest. Nat Cell Biol 9:160–170
Mullee LI, Morrison CG (2015) Centrosomes in the DNA damage response – the hub outside the centre. Chromosome Res doi:10.1007/s10577-015-9503-7
Musacchio A, Salmon ED (2007) The spindle-assembly checkpoint in space and time. Nat Rev Mol Cell Biol 8:379–393
Nam HJ, van Deursen JM (2014) Cyclin B2 and p53 control proper timing of centrosome separation. Nat Cell Biol 16:538–549
Nam HJ, Naylor RM, van Deursen JM (2015) Centrosome dynamics as a source of chromosomal instability. Trends Cell Biol 25:65–73
Nano M, Basto R (2015) The Janus soul of centrosomes: a paradoxical role in disease? Chromosome Res. In press
Nigg EA, Raff JW (2009) Centrioles, centrosomes, and cilia in health and disease. Cell 139:663–678
Nigg EA, Stearns T (2011) The centrosome cycle: centriole biogenesis, duplication and inherent asymmetries. Nat Cell Biol 13:1154–1160
Palazzo RE, Vogel JM, Schnackenberg BJ, Hull DR, Wu X (2000) Centrosome maturation. Curr Top Dev Biol 49:449–470
Piel M, Nordberg J, Euteneuer U, Bornens M (2001) Centrosome-dependent exit of cytokinesis in animal cells. Science 291:1550–1553
Poulton JS, Mu FW, Roberts DM, Peifer M (2013) APC2 and Axin promote mitotic fidelity by facilitating centrosome separation and cytoskeletal regulation. Development 140:4226–4236
Poulton JS, Cuningham JC, Peifer M (2014) Acentrosomal Drosophila epithelial cells exhibit abnormal cell division, leading to cell death and compensatory proliferation. Dev Cell 30:731–745
Prosser SL, Sahota NK, Pelletier L, Morrison CG, Fry AM (2015) Nek5 promotes centrosome integrity in interphase and loss of centrosome cohesion in mitosis. J Cell Biol 209:339–348
Rebollo E, Sampaio P, Januschke J, Llamazares S, Varmark H, Gonzalez C (2007) Functionally unequal centrosomes drive spindle orientation in asymmetrically dividing Drosophila neural stem cells. Dev Cell 12:467–474
Rieder CL, Maiato H (2004) Stuck in division or passing through: what happens when cells cannot satisfy the spindle assembly checkpoint. Dev Cell 7:637–651
Rodrigues-Martins A, Riparbelli M, Callaini G, Glover DM, Bettencourt-Dias M (2008) From centriole biogenesis to cellular function: centrioles are essential for cell division at critical developmental stages. Cell Cycle 7:11–16
Rusan NM, Peifer M (2007) A role for a novel centrosome cycle in asymmetric cell division. J Cell Biol 177:13–20
Sakurai H, Okado M, Ito F, Kawasaki K (2011) Anaphase DNA bridges induced by lack of RecQ5 in Drosophila syncytial embryos. FEBS Lett 585:1923–1928
Schmutz C, Spang A (2005) Knockdown of the centrosomal component SAS-5 results in defects in nuclear morphology in Caenorhabditis elegans. Eur J Cell Biol 84:75–82
Schoenfelder KP, Montague RA, Paramore SV, Lennox AL, Mahowald AP, Fox DT (2014) Indispensable pre-mitotic endocycles promote aneuploidy in the Drosophila rectum. Development 141:3551–3560
Schumacher JM, Ashcroft N, Donovan PJ, Golden A (1998) A highly conserved centrosomal kinase, AIR-1, is required for accurate cell cycle progression and segregation of developmental factors in Caenorhabditis elegans embryos. Development 125:4391–4402
Sibon OC, Kelkar A, Lemstra W, Theurkauf WE (2000) DNA-replication/DNA-damage-dependent centrosome inactivation in Drosophila embryos. Nat Cell Biol 2:90–95
Silkworth WT, Nardi IK, Paul R, Mogilner A, Cimini D (2012) Timing of centrosome separation is important for accurate chromosome segregation. Mol Biol Cell 23:401–411
Singh P, Ramdas Nair A, Cabernard C (2014) The centriolar protein Bld10/Cep135 is required to establish centrosome asymmetry in Drosophila neuroblasts. Curr Biol 24:1548–1555
Sir JH, Putz M, Daly O, Morrison CG, Dunning M, Kilmartin JV, Gergely F (2013) Loss of centrioles causes chromosomal instability in vertebrate somatic cells. J Cell Biol 203:747–756
Srsen V, Gnadt N, Dammermann A, Merdes A (2006) Inhibition of centrosome protein assembly leads to p53-dependent exit from the cell cycle. J Cell Biol 174:625–630
Stevens NR, Raposo AA, Basto R, St Johnston D, Raff JW (2007) From stem cell to embryo without centrioles. Curr Biol 17:1498–1503
Sunkel CE, Glover DM (1988) polo, a mitotic mutant of Drosophila displaying abnormal spindle poles. J Cell Sci 89(Pt 1):25–38
Takada S, Kelkar A, Theurkauf WE (2003) Drosophila checkpoint kinase 2 couples centrosome function and spindle assembly to genomic integrity. Cell 113:87–99
Tanenbaum ME, Medema RH (2010) Mechanisms of centrosome separation and bipolar spindle assembly. Dev Cell 19:797–806
Uetake Y, Loncarek J, Nordberg JJ, English CN, La Terra S, Khodjakov A, Sluder G (2007) Cell cycle progression and de novo centriole assembly after centrosomal removal in untransformed human cells. J Cell Biol 176:173–182
Ulrich E, Boehmelt G, Bird A, Beug H (1992) Immortalization of conditionally transformed chicken cells: loss of normal p53 expression is an early step that is independent of cell transformation. Genes Dev 6:876–887
Vaizel-Ohayon D, Schejter ED (1999) Mutations in centrosomin reveal requirements for centrosomal function during early Drosophila embryogenesis. Curr Biol 9:889–898
Varmark H, Llamazares S, Rebollo E, Lange B, Reina J, Schwarz H, Gonzalez C (2007) Asterless is a centriolar protein required for centrosome function and embryo development in Drosophila. Curr Biol 17:1735–1745
Wainman A, Buster DW, Duncan T, Metz J, Ma A, Sharp D, Wakefield JG (2009) A new Augmin subunit, Msd1, demonstrates the importance of mitotic spindle-templated microtubule nucleation in the absence of functioning centrosomes. Genes Dev 23:1876–1881
Wong YL, Anzola JV, Davis RL, Yoon M, Motamedi A, Kroll A, Seo CP, Hsia JE, Kim SK, Mitchell JW et al (2015) Cell biology. Reversible centriole depletion with an inhibitor of Polo-like kinase 4. Science 348:1155–1160
Yamashita YM, Mahowald AP, Perlin JR, Fuller MT (2007) Asymmetric inheritance of mother versus daughter centrosome in stem cell division. Science 315:518–521
Zhang Y, Foreman O, Wigle DA, Kosari F, Vasmatzis G, Salisbury JL, van Deursen J, Galardy PJ (2012) USP44 regulates centrosome positioning to prevent aneuploidy and suppress tumorigenesis. J Clin Invest 122:4362–4374
Zhang CZ, Spektor A, Cornils H, Francis JM, Jackson EK, Liu S, Meyerson M, Pellman D (2015) Chromothripsis from DNA damage in micronuclei. Nature 522:179–184
Acknowledgments
We thank Nasser M. Rusan, Mark Peifer, and Erich Kushner for critical comments. DAL is supported by a Lenfant Biomedical Postdoctoral Fellowship and a NHLBI Career Transition Award (1K22HL126922). JSP is supported by the Peifer lab grant NIH R01GM067236.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Responsible Editors: Daniela Cimini and Giulia Guarguaglini
Dorothy A. Lerit and John S. Poulton contributed equally to this work.
Rights and permissions
About this article
Cite this article
Lerit, D.A., Poulton, J.S. Centrosomes are multifunctional regulators of genome stability. Chromosome Res 24, 5–17 (2016). https://doi.org/10.1007/s10577-015-9506-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10577-015-9506-4