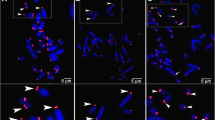
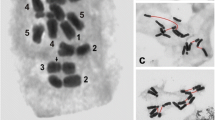
Abstract
Two closely related shrew species, Sorex granarius and Sorex araneus, in which Robertsonian rearrangements have played a primary role in karyotype evolution, present very distinct telomere length patterns. S. granarius displays hyperlong telomeres specifically associated with the short arms of acrocentrics, whereas telomere lengths in S. araneus are rather short and homogenous. Using a combined approach of chromosome and fibre FISH, modified Q-FISH, 3D-FISH, Ag-NOR staining and TRF analysis, we carried out a comparative analysis of telomeric repeats and rDNA distribution on chromosome ends of Sorex granarius. Our results show that rDNA sequences forming active nuclear organizing regions are interspersed with the long telomere tracts of all short arms of acrocentrics. These observations suggest that the major rearrangements that gave rise to today’s karyotype in S. granarius were accompanied by a profound reorganization of chromosome ends, which comprised extensive amplification of telomeric and rDNA repeats on the short arms of acrocentrics and finally contributed to the stabilization of telomeres. This is the first time that such telomeric structures have been observed in any mammalian species.
Similar content being viewed by others
References
Allsopp RC, Vaziri H, Patterson C et al. (1992) Telomere length predicts replicative capacity of human fibroblasts. Proc Natl Acad Sci USA 89: 10114–10118.
Allsopp RC, Chang E, Kashefi-Aazam M et al. (1995) Telomere shortening is associated with cell division in vitro and in vivo. Exp Cell Res 220: 194–200.
Blocher D, Kunhi M (1990) DNA double-strand break analysis by CHEF (clamped homogeneous electrical field) electrophoresis. Int J Radiat Biol 58: 23–34.
Cristofalo VJ, Lorenzini A, Allen RG, Torres C, Tresini M (2004) Replicative senescence: a critical review. Mech Ageing Dev 125: 827–848.
Der-Sarkissian H, Vergnaud G, Borde YM, Thomas G, Londono-Vallejo JA (2002) Segmental polymorphisms in the proterminal regions of a subset of human chromosomes. Genome Res 12: 1673–1678.
Dobigny G, Ozouf-Costaz C, Bonillo C, Volobouev V (2002) “Ag-NORs” are not always true NORs: new evidence in mammals. Cytogenet Genome Res 98: 75–77.
Dobigny G, Ozouf-Costaz C, Bonillo C, Volobouev V (2003) Evolution of rRNA gene clusters and telomeric repeats during explosive genome repatterning in TATERILLUS X (Rodentia Gerbillinae). Cytogenet Genome Res 103: 94–103.
Fumagalli L, Taberlet P, Stewart DT et al. (1999) Molecular phylogeny and evolution of Sorex shrews (Soricidae: insectivora) inferred from mitochondrial DNA sequence data. Mol Phylogenet Evol 11: 222–235.
Goodpasture C, Bloom SE (1975) Visualization of nucleolar organizer regions in mammalian chromosomes using silver staining. Chromosoma 53: 37–50.
Halkka L, Halkka O, Skaren U, Soderlund V (1974) Chromosome banding pattern in a polymorphic population of Sorex araneus from northeastern Finland. Hereditas 76: 305–314.
Hall KJ, Parker JS (1995) Stable chromosome fission associated with rDNA mobility. Chromosome Res 3: 417–422.
Harper L, Golubovskaya I, Cande WZ (2004) A bouquet of chromosomes. J Cell Sci 117: 4025–4032.
Hausser J, Fedyk S, Fredga K et al. (1994) Definition and nomenclature of chromosome races of Sorex araneus. Folia Zool 43: 1–9.
Hausser J, Fumagalli L, Taberlet P et al. (1998) Mitochondrial DNA evolution in shrews. In: Wojcik JM, Wolsan M, eds. Evolution of Shrews. Bialowieza: Mammal Research Institute, pp. 295–308.
Ijdo JW, Wells RA, Baldini A, Reeders ST (1991) Improved telomere detection using a telomere repeat probe (TTAGGG)n generated by PCR. Nucleic Acids Res 19: 4780.
Linardopoulou EV, Williams EM, Fan Y et al. (2005) Human subtelomeres are hot spots of interchromosomal recombination and segmental duplication. Nature 437: 94–100.
Liu WS, Fredga K (1999) Telomeric (TTAGGG)n sequences are associated with nucleolus organizer regions (NORs) in the wood lemming. Chromosome Res 7: 235–240.
Londono-Vallejo JA (2004) Telomere length heterogeneity and chromosome instability. Cancer Lett 212: 135–144.
Malygin AA, Graifer DM, Zenkova MA, Mamaev SV, Karpov GG (1992) Affinity modification of 80S ribosomes from human placenta by derivatives of tri- and hexauridylates as mRNA analogs. Mol Biol (Mosk) 26: 369–377.
Meyne J, Baker RJ, Hobart HH et al. (1990) Distribution of non-telomeric sites of the (TTAGGG)n telomeric sequence in vertebrate chromosomes. Chromosoma 99: 3–10.
Minina JM, Borodin PM, Searle JB, Volobouev V, Zhdanova NS (2007) Standard DAPI karyotype of the common shrew Sorex araneus L (Soricidae Eulipothla). Russian J Teriology (accepted for publication)
Olert J, Schmid M (1978) Comparative analysis of karyotypes in European shrew species. I The sibling species Sorex araneus and S. gemellus: Q-bands G-bands, and position of NORs. Cytogenet Cell Genet 20: 308–322.
Pich U, Fuchs J, Schubert I (1996) How do Alliaceae stabilize their chromosome ends in the absence of TTTAGGG sequences? Chromosome Res 4: 207–213.
Rakotoarisoa G, Hirai Y, Go Y et al. (2000) Chromosomal localization of 18S rDNA and telomere sequence in the aye-aye Daubentonia madagascariensis. Genes Genet Syst 75: 299–303.
Raudsepp T, Christensen K, Chowdhar BP (2000) Cytogenetics of donkey chromosomes: nomenclature proposal based on GTG-banded chromosomes and depiction of NORs and telomeric sites. Chromosome Res 8: 659–670.
Ray M (1979) Nucleolar organizing regions of normal Chinese hamster and CHW cell line chromosomes. Cytobios 25: 37–43.
Rogatcheva MB, Serdyukova NA, Biltueva LS et al. (1997) Localization of the genes for major ribosomal RNA on chromosomes of the house musk shrew Suncus murinus, at meiotic and mitotic cells by fluorescence in situ hybridization and silver staining. Genes Genet Syst 72: 215–218.
Rousselet J, Monti L, Auger-Rozenberg MA, Parker JS, Lemeunier F (2000) Chromosome fission associated with growth of ribosomal DNA in Neodiprion abietis (Hymenoptera: Diprionidae). Proc Biol Sci 267: 1819–1823.
Searle JB, Fedyk S, Fredga K, Hausser J, Volobouev V (1991) Nomenclature for the chromosomes of the common shrew (Sorex araneus). Mem Soc Vaud Sc Nat 19: 13–22.
Solovei I, Cavallo A, Schermelleh L et al. (2002) Spatial preservation of nuclear chromatin architecture during three-dimensional fluorescence in situ hybridization (3D-FISH). Exp Cell Res 276: 10–23.
Sybenga J (1999) What makes homologous chromosomes find each other in meiosis? A review and an hypothesis. Chromosoma 108: 209–219.
Verma RS, Babu A (1995) Human Chromosomes. Principles and Techniques. New York: McGraw-Hill.
Weipoltshammer K, Schofer C, Almeder M et al. (1999) Intranuclear anchoring of repetitive DNA sequences: centromeres, telomeres, and ribosomal DNA. J Cell Biol 147: 1409–1418.
Wojcik JM, Searle JB (1988) The chromosome complement of Sorex granarius—the ancestral karyotype of the common shrew (Sorex araneus)? Heredity 61(Pt 2): 225–229.
Zhdanova NS, Karamisheva TV, Minina J et al. (2005) Unusual distribution pattern of telomeric repeats in the shrews Sorex araneus and Sorex granarius. Chromosome Res 13: 617–625.
Zhu L, Hathcock KS, Hande P et al. (1998) Telomere length regulation in mice is linked to a novel chromosome locus. Proc Natl Acad Sci USA 95: 8648–8653.
Zijlmans JM, Martens UM, Poon SS et al. (1997) Telomeres in the mouse have large inter-chromosomal variations in the number of T2AG3 repeats. Proc Natl Acad Sci USA 94: 7423–7428.
Zima J, Lukacova L, Macholan M (1998) Chromosomal evolution in shrews. In: Wojcik JM, Wolsan M, eds. Evolution of Shrews. Bialowieza: Mammal Research Institute, pp 175–218.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhdanova, N.S., Minina, J.M., Karamisheva, T.V. et al. The very long telomeres in Sorex granarius (Soricidae, Eulipothyphla) contain ribosomal DNA. Chromosome Res 15, 881–890 (2007). https://doi.org/10.1007/s10577-007-1170-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10577-007-1170-x