Abstract
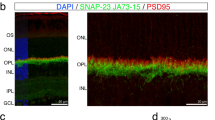
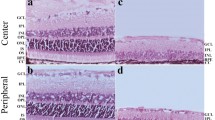
Visually guided regulation is a sophisticated and active process, whereby sensory input helps to shape ocular development. Here, we sought to investigate the potential involvement of SorCS1, an important protein in synaptic transmission in neuron, in retinal development. A form-deprivation (FD) rat model was established. Ocular variations induced by FD were examined, including changes to eye axial length and retinal thickness. Scotopic electroretinogram (ERG) was used to examine retinal function. RD-PCR assays were screened for differentially expressed genes in FD rat eyes. Immunofluorescence staining identified the expression pattern and localization of SorCS1 in rat retina, with or without FD treatment. Additionally, primary retinal neural cells were cultured and incubated with or without a light–dark cycle, and western blot and real-time PCR assays were used to examine the expression of SorCS1. Retinal neural cells were treated with recombinant SorCS1 (h-SorCS1) coated with beads in serum-free conditions to test for effects on cellular physiology and expression of neurotransmitters involved in visual development. To monitor cell viability, a CCK8 assay was employed. Our data demonstrated that FD led to ocular axial elongation and retinal thinning. ERG tests showed FD impaired electrophysiological function in rat. An age-related expression pattern of SorCS1 was observed in the rat retina, and SorCS1 was significantly up-regulated in the FD rat retina. In addition, in vitro evidence suggested a strong correlation between light exposure and SorCS1 expression. Furthermore, treatment of retinal neural cells with h-SorCS1-beads promoted cell viability, neurite outgrowth, and up-regulation of inhibitory neurotransmitter expression, which implies that over-expression of SorCS1 may cause abnormal retinal development. Our findings suggest that SorCS1 is involved in the physiological processes of light/visually guided ocular growth.





Similar content being viewed by others
References
Bowrey HE, Metse AP, Leotta AJ, Zeng G, McFadden SA (2015) The relationship between image degradation and myopia in the mammalian eye. CLIN EXP OPTOM 98:555–563
Contini M, Raviola E (2003) GABAergic synapses made by a retinal dopaminergic neuron. Proc Natl Acad Sci USA 100:1358–1363
Escaño MF, Fujii S, Sekiya Y, Yamamoto M, Negi A (2000) Expression of Sonic hedgehog and retinal opsin genes in experimentally-induced myopic chick eyes. Exp Eye Res 71:459–467
Fang F, Pan M, Yan T, Tao Y, Wu H, Liu X, Qu J, Zhou X (2013) The role of cGMP in ocular growth and the development of form-deprivation myopia in guinea pigs. Invest Ophthalmol Vis Sci 54:7887–7902
Feldkaemper M, Schaeffel F (2013) An updated view on the role of dopamine in myopia. Exp Eye Res 114:106–119
Godinho L, Mumm JS, Williams PR et al (2005) Targeting of amacrine cell neurites to appropriate synaptic laminae in the developing zebrafish retina. Development 132(22):5069–5079
Graf ER, Zhang XZ, Jin SX et al (2004) Neurexins induce differentiation of GABA and glutamate postsynaptic specializations via neuroligins. Cell 119(7):1013–1026
He M, Xiang F, Zeng Y et al (2015) Effect of time spent outdoors at school on the development of myopia among children in china: a randomized clinical trial. JAMA 314(11):1142–1148
Hermey G (2009) The Vps10p-domain receptor family. Cell Mol Life Sci 66:2677–2689
Hermey G, Riedel IB, Hampe W, Schaller HC, Hermans-Borgmeyer I (1999) Identification and characterization of SorCS, a third member of a novel receptor family. Biochem Biophys Res Commun 266:347–351
Hermey G, Schaller HC, Hermans-Borgmeyer I (2001a) Transient expression of SorCS in developing telencephalic and mesencephalic structures of the mouse. NeuroReport 12:29–32
Hermey G, Riedel IB, Rezgaoui M, Westergaard UB, Schaller C, Hermans-Borgmeyer I (2001b) SorCS1, a member of the novel sorting receptor family, is localized in somata and dendrites of neurons throughout the murine brain. Neurosci Lett 313:83–87
Hermey G, Plath N, Hubner CA, Kuhl D, Schaller HC, Hermans-Borgmeyer I (2004) The three sorCS genes are differentially expressed and regulated by synaptic activity. J Neurochem 88:1470–1476
Houston SK, Bourne TD, Lopes MB, Ghazi NG (2009) Bilateral massive retinal gliosis associated with retinopathy of prematurity. Arch Pathol Lab Med 133(8):1242–1245
Huang J, Qu XM, Chu RY (2011) Expressions of cellular retinoic acid binding proteins I and retinoic acid receptor-beta in the guinea pig eyes with experimental myopia. Int J Ophthalmol 4:131–136
Huang F, Yan T, Shi F et al (2014) Activation of dopamine d2 receptor is critical for the development of form-deprivation myopia in the c57bl/6 mouse. Investig Ophthalmol Vis Sci 55(9):5537–5544
Jacobson SG, Mohindra I, Held R (1983) Monocular visual form deprivation in human infants. Documenta Ophthalmol 55(3):199–211
Jones-Jordan LA, Sinnott LT, Cotter SA et al (2012) Time outdoors, visual activity, and myopia progression in juvenile-onset myopes. Invest Ophthalmol Vis Sci 53:7169–7175
Karouta C, Ashby RS (2014) Correlation between light levels and the development of deprivation myopia. Invest Ophthalmol Vis Sci 56:299–309
Kay JN, Roeser T, Mumm JS et al (2004) Transient requirement for ganglion cells during assembly of retinal synaptic layers. Development 131(6):1331–1342
Ma M, Zhang Z, Du E, Zheng W, Gu Q, Xu X, Ke B (2014) Wnt signaling in form deprivation myopia of the mice retina. PLoS ONE 9:e91086
Read SA, Collins MJ, Vincent SJ (2015) Light exposure and eye growth in childhood. Invest Ophthalmol Vis Sci 56(11):6779–6787
Reitz C (2015) The role of the retromer complex in aging-related neurodegeneration: a molecular and genomic review. Mol Genet Genomics 290:413–427
Rose KA, Morgan IG, Ip J, Kifley A, Huynh S, Smith W, Mitchell P (2008) Outdoor activity reduces the prevalence of myopia in children. Ophthalmology 115:1279–1285
Rymer J, Wildsoet CF (2005) The role of the retinal pigment epithelium in eye growth regulation and myopia: a review. Vis Neurosci 22:251–261
Savas JN, Ribeiro LF, Wierda KD et al (2015) The sorting receptor SorCS1 regulates trafficking of neurexin and AMPA receptors. Neuron 87:764–780
Sun ZH, Ma WL, Zhang B, Peng YF, Zheng WL (2005) Application of restriction display PCR technique in the preparation of cDNA microarray probes. World J Gastroenterol 11:7579–7584
Tian N, Copenhagen ADR (2001) Visual deprivation alters development of synaptic function in inner retina after eye opening. Neuron 32(3):439–449
Tian N, Xu H, Wang P (2015) Dopamine d2 receptors preferentially regulate the development of light responses of the inner retina. Eur J Neurosci 41(1):17–30
Wallman J, Winawer J (2004) Homeostasis of eye growth and the question of myopia. Neuron 43:447–468
Wallman J, Turkel J, Trachtman J (1978) Extreme myopia produced by modest change in early visual experience. Science 201(4362):1249–1251
Wallman J, Gottlieb MD, Rajaram V, Fugate-Wentzek LA (1987) Local retinal regions control local eye growth and myopia. Science 237:73–77
Wang J, Zhu C, Xu Y, Liu B, Wang M, Wu K (2011) Development and expression of amyloid-β peptide 42 in retinal ganglion cells in rats. Chin J Anat 294(8):1401–1405
Wildsoet CF (2010) Active emmetropization—evidence for its existence and ramifications for clinical practice. Ophthalmic Physiol Opt 17(4):279–290
Wildsoet CF, Schmid KL (2000) Optical correction of form deprivation myopia inhibits refractive recovery in chick eyes with intact or sectioned optic nerves. Vision Res 40(23):3273–3282
Witkovsky P (2004) Dopamine and retinal function. Doc Ophthalmol 108:17–40
Zhang W (2012) SORCS1 and APOE polymorphisms interact to confer risk for late-onset Alzheimer’s disease in a Northern Han Chinese population. Brain Res 1448:111–116
Funding
This work was supported by National Natural Science Foundation of China (Grant Nos. 81670848 and 81470626).
Author information
Authors and Affiliations
Contributions
KMY and JZ conceived and designed the experiments. PC, JZ, XXC, and YY performed the experiments. PC, LJX, JQ, and QYW analyzed the data. JG and JZ provided material support. PC, JZY, and JZ wrote the paper.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
10571_2019_740_MOESM1_ESM.tif
Supplementary material 1 Supplemental data 1: The rabbit mAb IgG XP® Isotype Control for Socs1 specificity in rat retinal tissue. (TIFF 1716 kb)
Rights and permissions
About this article
Cite this article
Chen, P., Xu, L., Zhang, J. et al. Up-Regulation of SorCS1, an Important Sorting Receptor, in the Retina of a Form-Deprivation Rat Model. Cell Mol Neurobiol 40, 395–405 (2020). https://doi.org/10.1007/s10571-019-00740-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10571-019-00740-1