Abstract
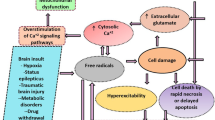
It is of great importance to explore the development of epileptogenesis, and the adenosine and adenosine kinase (ADK) system seems to play a key role in this process. The aim of this study is to explore the dynamic changes of astrocytes and adenosine signaling during epileptogenesis in rat hippocampus in a post-status epileptogenesis (SE) model. Rat SE models were built and killed for experiments at 1 day (acute phase of epileptogenesis), 5 days (latent phase), 4 weeks (chronic phase), and 8 weeks (late chronic phase of epileptogenesis) after SE induction. Immunofluorescence staining, high-performance liquid chromatography, and Western blotting were performed to assess changes of astrocytes, adenosine, ADK, and ADK receptors (including A1R, A2aR, A2bR, and A3R) in hippocampus. The expression level of glial fibrillary acidic protein significantly increased from latent to late chronic phase. The concentration of adenosine sharply increased in acute phase and gradually decreased in the remaining phases of post-SE, being significantly lower than in the control group in late chronic phase. Protein levels of A1R and A2aR in post-SE models increased in acute phase, whereas A2bR and A3R protein expression decreased in latent phase, chronic phase, and late chronic phase following post-SE epileptogenesis. Protein expression of ADK significantly increased during latent phase, chronic phase, and late chronic phase of post-SE epileptogenesis. In conclusion, the levels of adenosine and protein expression of A1R and A2R significantly increased during acute phase of post-SE. During the remaining phases of post-SE epileptogenesis, there was imbalance among astrocytes, adenosine, adenosine receptors, and ADK. Regulation of the ADK/adenosine system may provide potential treatment strategies for epileptogenesis.




Similar content being viewed by others
References
Arisi GM, Ruch M, Foresti ML, Mukherjee S, Ribak CE, Shapiro LA (2011) Astrocyte alterations in the hippocampus following pilocarpine-induced seizures in aged rats. Aging Dis 2(4):294–300
Binder DK, Steinhäuser C (2017) Role of astrocyte dysfunction in epilepsy. Reference module in neuroscience and biobehavioral psychology. Elsevier, Amsterdam. https://doi.org/10.1016/B978-0-12-809324-5.00071-7
Boison D (2008) The adenosine kinase hypothesis of epileptogenesis. Progr Neurobiol 84(3):249–262. https://doi.org/10.1016/j.pneurobio.2007.12.002
Boison D (2013) Role of adenosine in status epilepticus: a potential new target? Epilepsia 54(Suppl 6):20–22. https://doi.org/10.1111/epi.12268
Boison D, Masino SA, Geiger JD (2011) Homeostatic bioenergetic network regulation - a novel concept to avoid pharmacoresistance in epilepsy. Expert Opin Drug Discov 6(7):713–724. https://doi.org/10.1517/17460441.2011.575777
Curia G, Longo D, Biagini G, Jones RS, Avoli M (2008) The pilocarpine model of temporal lobe epilepsy. J Neurosci Methods 172(2):143–157. https://doi.org/10.1016/j.jneumeth.2008.04.019
Diogenes MJ, Neves-Tome R, Fucile S, Martinello K, Scianni M, Theofilas P, Lopatar J, Ribeiro JA, Maggi L, Frenguelli BG, Limatola C, Boison D, Sebastiao AM (2014) Homeostatic control of synaptic activity by endogenous adenosine is mediated by adenosine kinase. Cerebral Cortex 24(1):67–80. https://doi.org/10.1093/cercor/bhs284
do Nascimento AL, Dos Santos NF, Campos Pelagio F, Aparecida Teixeira S, de Moraes Ferrari EA, Langone F (2012) Neuronal degeneration and gliosis time-course in the mouse hippocampal formation after pilocarpine-induced status epilepticus. Brain Res 1470:98–110. https://doi.org/10.1016/j.brainres.2012.06.008
Dragunow M (1991) Adenosine and seizure termination. Ann Neurol 29(5):575. https://doi.org/10.1002/ana.410290524
During MJ, Spencer DD (1992) Adenosine: a potential mediator of seizure arrest and postictal refractoriness. Ann Neurol 32(5):618–624. https://doi.org/10.1002/ana.410320504
Engel J Jr (1996) Clinical evidence for the progressive nature of epilepsy. Epilepsy Res Suppl 12:9–20
Gibbons MB, Smeal RM, Takahashi DK, Vargas JR, Wilcox KS (2013) Contributions of astrocytes to epileptogenesis following status epilepticus: opportunities for preventive therapy? Neurochem Int 63(7):660–669. https://doi.org/10.1016/j.neuint.2012.12.008
Giulia C, Daniela L, Giuseppe B, Jones RSG, Massimo A (2008) The pilocarpine model of temporal lobe epilepsy. J Neurosci Methods 172(2):143–157
Gomes CV, Kaster MP, Tomé AR, Agostinho PM, Cunha RA (2011) Adenosine receptors and brain diseases: neuroprotection and neurodegeneration. Biochim Biophys Acta (BBA) 1808(5):1380–1399. https://doi.org/10.1016/j.bbamem.2010.12.001
Haut SR, Veliskova J, Moshe SL (2004) Susceptibility of immature and adult brains to seizure effects. Lancet Neurol 3(10):608–617. https://doi.org/10.1016/s1474-4422(04)00881-6
Huicong K, Zheng X, Furong W, Zhouping T, Feng X, Qi H, Xiaoyan L, Xiaojiang H, Na Z, Ke X, Zheng Z, Suiqiang Z (2013) The imbalanced expression of adenosine receptors in an epilepsy model corrected using targeted mesenchymal stem cell transplantation. Mol Neurobiol 48(3):921–930. https://doi.org/10.1007/s12035-013-8480-0
Kovacs Z, Kekesi KA, Juhasz G, Dobolyi A (2014) The antiepileptic potential of nucleosides. Curr Med Chem 21(6):788–821
Lauritzen F, Heuser K, de Lanerolle NC, Lee TS, Spencer DD, Kim JH, Gjedde A, Eid T, Bergersen LH (2012a) Redistribution of monocarboxylate transporter 2 on the surface of astrocytes in the human epileptogenic hippocampus. Glia 60(7):1172–1181. https://doi.org/10.1002/glia.22344
Lauritzen F, Heuser K, de Lanerolle NC, Lee TS, Spencer DD, Kim JH, Gjedde A, Eid T, Bergersen LH (2012b) Redistribution of monocarboxylate transporter 2 on the surface of astrocytes in the human epileptogenic hippocampus. Glia 60(7):1172
Lemos T, Cavalheiro EA (1995) Suppression of pilocarpine-induced status epilepticus and the late development of epilepsy in rats. Exp Brain Res 102(3):423–428
Luo Y, Hu Q, Zhang Q, Hong S, Tang X, Chen L, Jiang L (2015a) Alterations in hippocampal myelin and oligodendrocyte precursor cells during epileptogenesis. Brain Res 1627:154
Luo Y, Hu Q, Zhang Q, Hong S, Tang X, Cheng L, Jiang L (2015b) Alterations in hippocampal myelin and oligodendrocyte precursor cells during epileptogenesis. Brain Res 1627:154
Mazzuferi M, Palma E, Martinello K, Maiolino F, Roseti C, Fucile S, Fabene PF, Schio F, Pellitteri M, Sperk G (2010) Enhancement of GABA current run-down in the hippocampus occurs at the first spontaneous seizure in a model of temporal lobe epilepsy. Proc Natl Acad Sci USA 107(7):3180–3185
Pelvig DP, Pakkenberg H, Stark AK, Pakkenberg B (2008) Neocortical glial cell numbers in human brains. Neurobiol Aging 29(11):1754–1762. https://doi.org/10.1016/j.neurobiolaging.2007.04.013
Rebola N, Pinheiro PC, Oliveira CR, Malva JO, Cunha RA (2003) Subcellular localization of adenosine A(1) receptors in nerve terminals and synapses of the rat hippocampus. Brain Res 987(1):49–58
Sachdeva S, Gupta M (2013) Adenosine and its receptors as therapeutic targets: An overview. Saudi Pharm J 21(3):245–253. https://doi.org/10.1016/j.jsps.2012.05.011
Shen HY, Sun H, Hanthorn MM, Zhi Z, Lan JQ, Poulsen DJ, Wang RK, Boison D (2014) Overexpression of adenosine kinase in cortical astrocytes and focal neocortical epilepsy in mice. J Neurosurg 120(3):628–638. https://doi.org/10.3171/2013.10.jns13918
Turski WA, Cavalheiro EA, Schwarz M, Czuczwar SJ, Kleinrok Z, Turski L (1983) Limbic seizures produced by pilocarpine in rats: Behavioural, electroencephalographic and neuropathological study. Behav Brain Res 9(3):315–335
Ye Y, Xiong J, Hu J, Kong M, Cheng L, Chen H, Li T, Jiang L (2013) Altered hippocampal myelinated fiber integrity in a lithium-pilocarpine model of temporal lobe. epilepsy: A histopathological and stereological investigation. Brain Res 1522(30):76–87
Yuzlenko O, Kiec-Kononowicz K (2006) Potent adenosine A1 and A2A receptors antagonists: recent developments. Curr Med Chem 13(30):3609–3625
Acknowledgements
This work was supported by the National Natural Science Foundation of China (NSFC grant 81371452), Natural Science Foundation of Chongqing (CSTC 2013jjB0031; CSTC 2015jcyjA10091), Research Fund for the Doctoral Program of Higher Education of China (20125503110011), and Young and Middle-Age High-Level Medical Reserved Personnel Training Project Foundation of Chongqing.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflicts of interest.
Rights and permissions
About this article
Cite this article
Hong, S., Li, T., Luo, Y. et al. Dynamic Changes of Astrocytes and Adenosine Signaling in Rat Hippocampus in Post-status Epilepticus Model of Epileptogenesis. Cell Mol Neurobiol 38, 1227–1234 (2018). https://doi.org/10.1007/s10571-018-0590-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10571-018-0590-9