Abstract
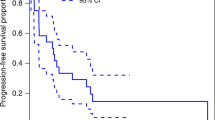
Patients harboring germline mutations in the succinate dehydrogenase complex subunit B (SDHB) gene present with pheochromocytomas and paragangliomas (PPGL) that are more likely malignant and clinically aggressive. The combination chemotherapy cyclophosphamide, vincristine, and dacarbazine (CVD) was retrospectively evaluated in patients with SDHB-associated metastatic PPGL.Query Twelve metastatic PPGL patients harboring SDHB mutations/polymorphisms with undetectable SDHB immunostaining were treated with CVD. CVD therapy consisted of 750 mg/m2 cyclophosphamide with 1.4 mg/m2 vincristine on day 1 and 600 mg/m2 dacarbazine on days 1 and 2, every 21–28 days. Treatment outcome was determined by RECIST criteria as well as determination of response duration and progression-free and overall survivals. A median of 20.5 cycles (range 4–41) was administered. All patients had tumor reduction (12–100% by RECIST). Complete response was seen in two patients, while partial response was observed in 8. The median number of cycles to response was 5.5. Median duration of response was 478 days, with progression-free and overall survivals of 930 and 1190 days, respectively. Serial [18F]-fluorodeoxyglucose positron emission tomography and computed tomography imaging demonstrated continued incremental reduction in maximal standardized uptake values (SUVmax) values in 26/30 lesions. During treatment administration, the median SUV decreased from > 25 to < 6, indicating the efficacy of chemotherapy over a prolonged period of time. Prolonged therapy results in continued incremental tumor reduction, and is consistent with persistent drug sensitivity. CVD chemotherapy is recommended to be considered part of the initial management in patients with metastatic SDHB-related PPGL.



Similar content being viewed by others
References
Agarwal G, Sadacharan D, Kappor A et al (2011) Cardiovascular dysfunction and catecholamine cardiomyopathy in pheochromocytoma patients and their reversal following surgical cure: results of a prospective case-control study. Surgery 150:1202–1211
Amar L, Bertherat J, Baudin E et al (2005) Genetic testing in pheochromocytoma or functional paraganglioma. J Clin Oncol 23:8812–8818
Andersen GS, Toftdahl DB, Lund JO et al (1988) The incidence rate of phaeochromocytoma and Conn’s syndrome in Denmark, 1977–1981. J Hum Hypertens 2:187–189
Averbuch SD, Steakley CS, Young RC et al (1988) Malignant pheochromocytoma: effective treatment with a combination of cyclophosphamide, vincristine, and dacarbazine. Ann Intern Med 109:267–273
Bausch B, Wellner U, Bausch D et al (2014) Long-term prognosis of patients with pediatric pheochromocytoma. Endocr Relat Cancer 21:17–25
Benn DE, Gimenez-Roqueplo AP, Reilly JR et al (2006) Clinical presentation and penetrance of pheochromocytoma/paraganglioma syndromes. J Clin Endocrinol Metab 91:827–836
Bravo EL (1991) Pheochromocytoma: new concepts and future trends. Kidney Int 40:544–556
Brouwers FM, Eisenhofer G, Tao JJ et al (2006) High frequency of SDHB germline mutations in patients with malignant catecholamine-producing paragangliomas: implications for genetic testing. J Clin Endocrinol Metab 91:4505–4509
Edström Elder E, Hjelm Skog AL, Höög A et al (2003) The management of benign and malignant pheochromocytoma and abdominal paraganglioma. Eur J Surg Oncol 29:278–283
Eisenhauer EA, Therasse P, Bogaerts J et al (2009) New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 45:228–247
Eisenhofer G, Tischler AS (2014) Neuroendocrine cancer: closing the GAPP on predicting metastases. Nat Rev Endocrinol 10:315–316
Eisenhofer G, Bornstein SR, Brouwers FM et al (2004) Malignant pheochromocytoma: current status and initiatives for future progress. Endocr Relat Cancer 11:423–436
Eisenhofer G, Lenders JW, Siegert G et al (2012) Plasma methoxytyramine: a novel biomarker of metastatic pheochromocytoma and paraganglioma in relation to established risk factors of tumour size, location and SDHB mutation status. Eur J Cancer 48:1739–1749
Fernandez-Calvet L, Garcia-Mayor RV (1994) Incidence of pheochromocytoma in South Galicia, Spain. J Intern Med 236:675–677
Glodny B, Winde G, Herwig R et al (2001) Clinical differences between benign and malignant pheochromocytomas. Endocr J 48:151–159
Goldstein RE, O’Neill JA Jr, Holcomb GW III et al (1999) Clinical experience over 48 years with pheochromocytoma. Ann Surg 229:755–764 discussion 764–766
Hartley L, Perry-Keene D (1985) Phaeochromocytoma in Queensland—1970–83. Aust N Z J Surg 55:471–475
Hawkins MM, Wilson LM, Stovall MA et al (1992) Epipodophyllotoxins, alkylating agents, and radiation and risk of secondary leukaemia after childhood cancer. BMJ 304:951–958
Hescot S, Leboulleux S, Amar L et al (2013) One-year progression-free survival of therapy-naive patients with malignant pheochromocytoma and paraganglioma. J Clin Endocrinol Metab 98:4006–4012
Hijiya N, Ness KK, Ribeiro RC et al (2009) Acute leukemia as a secondary malignancy in children and adolescents: current findings and issues. Cancer 115:23–25
Huang H, Abraham J, Hung E et al (2008) Treatment of malignant pheochromocytoma/paraganglioma with cyclophosphamide, vincristine, and dacarbazine: recommendation from a 22-year follow-up of 18 patients. Cancer 113:2020–2028
Korpershoek E, Favier J, Gaal J et al (2011) SDHA immunohistochemistry detects germline SDHA gene mutations in apparently sporadic paragangliomas and pheochromocytomas. J Clin Endocrinol Metab 96:E1472–E1476
Lenders JW, Eisenhofer G, Mannelli M et al (2005) Phaeochromocytoma. Lancet 35(366):665–675
Liu S, Wicha MS (2010) Targeting breast cancer stem cells. J Clin Oncol 28:4006–4012
Martucci VL, Pacak K (2014) Pheochromocytoma and paraganglioma: diagnosis, genetics, management, and treatment. Curr Probl Cancer 38:7–41
Neumann HP, Pawlu C, Peczkowska M et al (2004) Distinct clinical features of paraganglioma syndromes associated with SDHB and SDHD gene mutations. JAMA 292:943–951
Niemeijer ND, Alblas G, van Hulsteijn LT et al (2014) Chemotherapy with cyclophosphamide, vincristine and dacarbazine for malignant paraganglioma and pheochromocytoma: systematic review and meta-analysis. Clin Endocrinol (Oxf) 81:642–651
O’Riordain DS, Young WF Jr, Grant CS et al (1996) Clinical spectrum and outcome of functional extraadrenal paraganglioma. World J Surg 20:916–921 discussion 922
Pacak K (2007) Preoperative management of the pheohromocytoma patient. J Clin Endocrinol Metab 92:4069–4079
Plouin PF, Chatellier G, Fofol I et al (1997) Tumor recurrence and hypertension persistence after successful pheochromocytoma operation. Hypertension 29:11331139
Plouin PF, Duclos JM, Soppelsa F et al (2001) Factors associated with perioperative morbidity and mortality in patients with pheochromocytoma: analysis of 165 operations at a single center. J Clin Endocrinol Metab 86:1480–1486
Shen WT, Grogan R, Vriens M et al (2010) One hundred two patients with pheochromocytoma treated at a single institution since the introduction of laparoscopic adrenalectomy. Arch Surg 145:893–897
Srirangalingam U, Walker L, Khoo B et al (2008) Clinical manifestations of familial paraganglioma and phaeochromocytomas in succinate dehydrogenase B (SDH-B) gene mutation carriers. Clin Endocrinol (Oxf) 69:587–596
Stenström G, Svärdsudd K (1986) Pheochromocytoma in Sweden 1958–1981. An analysis of the national cancer registry data. Acta Med Scand 220:225–232
Stolk RF, Bakx C, Mulder J et al (2013) Is the excess cardiovascular morbidity in pheochromocytoma related to blood pressure or to catecholamines? J Clin Endocrinol Metab 98:1100–1106
Thompson LD (2002) Pheochromocytoma of the adrenal gland scaled score (PASS) to separate benign from malignant neoplasms: a clinicopathologic and immunophenotypic study of 100 cases. Am J Surg Pathol 26:551–566
Timmers HJ, Kozupa A, Eisenhofer G et al (2007a) Clinical presentations, biochemical phenotypes, and genotype-phenotype correlations in patients with succinate dehydrogenase subunit B-associated pheochromocytomas and paragangliomas. J Clin Endocrinol Metab 92:779–786
Timmers HJ, Kozupa A, Chen CC et al (2007b) Superiority of fluorodeoxyglucose positron emission tomography to other functional imaging techniques in the evaluation of metastatic SDHB-associated pheochromocytoma and paraganglioma. J Clin Oncol 25:2262–2269
Tsujimoto G, Manger WM, Hoffman BB (1984) Desensitization of beta-adrenergic receptors by pheochromocytoma. Endocrinology 114:1272–1278
Weisenthal LM, Lippman ME (1985) Clonogenic and nonclonogenic in vitro chemosensitivity assays. Cancer Treat Rep 69:615–632
Wu D, Tischler AS, Lloyd RV et al (2009) Observer variation in the application of the pheochromocytoma of the adrenal gland scaled score. Am J Surg Pathol 33:599–608
Zelinka T, Eisenhofer G, Pacak K (2007) Pheochromocytoma as a catecholamine producing tumor: implications for clinical practice. Stress 10:195–203
Funding
This work was supported by the Intramural Research Program of the National Institutes of Health; the Eunice Kennedy Shriver National Institute of Child Health and Human Development; and the German Research Foundation (DFG) (Grant Number DA 1630/1-1 to RD).
Author information
Authors and Affiliations
Contributions
IJ, KP, and TF designed the study. MV, RD, KIW, KA, AMV, SB, MSP, and JCR were involved in data collection, analysis, and interpretation. All authors have read and approve the final version of this manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical Approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the Institutional Review Board of the National Cancer Institute of the National Institutes of Health and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. This article does not contain any studies with animals performed by any of the authors.
Informed Consent
Informed consent was obtained from all individual participants included in the study.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Jawed, I., Velarde, M., Därr, R. et al. Continued Tumor Reduction of Metastatic Pheochromocytoma/Paraganglioma Harboring Succinate Dehydrogenase Subunit B Mutations with Cyclical Chemotherapy. Cell Mol Neurobiol 38, 1099–1106 (2018). https://doi.org/10.1007/s10571-018-0579-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10571-018-0579-4