Abstract
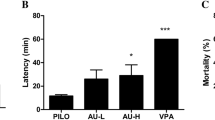
Epilepsy is one of the prevalent and major neurological disorders, and approximately one-third of the individuals with epilepsy experience seizures that do not respond well to available medications. We investigated whether oxysophocarpine (OSC) had anticonvulsant and neuroprotective property in the pilocarpine (PILO)-treated mice. Thirty minutes prior to the PILO injection, the mice were administrated with OSC (20, 40, and 80 mg/kg) once. Seizures and electroencephalography (EEG) were observed, and then the mice were killed for Nissl and Fluoro-jade B (FJB) staining. The oxidative stress was measured at 24 h after convulsion. Western blot analysis was used to examine the expressions of the Bax, Bcl-2, and Caspase-3. In this study, we found that pretreatment with OSC (40, 80 mg/kg) significantly delayed the onset of the first convulsion and status epilepticus (SE) and reduced the incidence of SE and mortality. Analysis of EEG recordings revealed that OSC (40, 80 mg/kg) significantly reduced epileptiform discharges. Furthermore, Nissl and FJB staining showed that OSC (40, 80 mg/kg) attenuated the neuronal cell loss and degeneration in hippocampus. In addition, OSC (40, 80 mg/kg) attenuated the changes in the levels of Malondialdehyde (MDA) and strengthened glutathione peroxidase and catalase activity in the hippocampus. Western blot analysis showed that OSC (40, 80 mg/kg) significantly decreased the expressions of Bax, Caspase-3 and increased the expression of Bcl-2. Collectively, the findings of this study indicated that OSC exerted anticonvulsant and neuroprotective effects on PILO-treated mice. The beneficial effects should encourage further studies to investigate OSC as an adjuvant in epilepsy, both to prevent seizures and to protect neurons in brain.






Similar content being viewed by others
References
Cavalheiro E, Silva D, Turski W, Calderazzo-Filho L, Bortolotto Z, Turski L (1987) The susceptibility of rats to pilocarpine-induced seizures is age-dependent. Dev Brain Res 37:43–58
Covolan L, Mello L (2000) Temporal profile of neuronal injury following pilocarpine or kainic acid-induced status epilepticus. Epilepsy Res 39:133–152
Dal-Pizzol F, Klamt F, Vianna M, Schröder N, Quevedo J, Benfato MS, Moreira JC, Walz R (2000) Lipid peroxidation in hippocampus early and late after status epilepticus induced by pilocarpine or kainic acid in Wistar rats. Neurosci Lett 291:179–182
DeGiorgio C, Han C, Xu C (1992) Hippocampal pyramidal cell loss in human status epilepticus. Epilepsia 33:23–27
Delorenzo R, Sun D, Deshpande L (2005) Cellular mechanisms underlying acquired epilepsy: the calcium hypothesis of the induction and maintainance of epilepsy. Pharmacol Ther 105:229–266
Folbergrova J (2013) Oxidative stress in immature brain following experimentally-induced seizures. Physiol Res 62:S39–S48
Freitas R, Sousa F, Vasconcelos S, Viana G, Fonteles M (2004) Pilocarpine-induced status epilepticus in rats: lipid peroxidation level, nitrite formation, GABAergic and glutamatergic receptor alterations in the hippocampus, striatum and frontal cortex. Pharmacol Biochem Behav 78:327–332
Freitas R, Vasconcelos S, Souza F, Viana G, Fonteles M (2005) Oxidative stress in the hippocampus after pilocarpine-induced status epilepticus in Wistar rats. FEBS J 272:1307
Fujikawa DG (2005) Prolonged seizures and cellular injury: understanding the connection. Epilepsy Behav 7:S3–S11
Galleano M, Puntarulo S (1995) Role of antioxidants on the erythrocytes resistance to lipid peroxidation after acute iron overload in rats. Biochim Biophys Acta 1271:321–326
Hui L, Pei D, Zhang Q, Guan Q, Zhang G (2005) The neuroprotection of insulin on ischemicn brain injury in rat hippocampus through negative regulation of JNK signaling pathway by PI3K/Akt activation. Brain Res 1052:1–9
Kamida T, Fujiki M, Ooba H, Anan M, Abe T, Kobayashi H (2009) Neuroprotective effects of edaravone, a free radical scavenger, on the rat hippocampus after pilocarpine-induced status epilepticus. Seizure 18:71–75
Katchanov J, Birbeck GL (2012) Epilepsy care guidelines for low- and middle- income countries: from WHO mental health GAP to national programs. BMC Med 10:107
Kim D, Cho K, Moon S, Kim Y, Kim D, Choi J, Chung H (2005) Cytoprotective mechanism of baicalin against endothelial cell damage by peroxynitrite. J Pharm Pharmacol 57:1581–1590
Liang L, Patel M (2006) Seizure-induced changes in mitochondrial redox status. Free Radic Biol Med 40:316–322
Liu C, Wu H, Kao S, Wei Y (1997) Phenytoin-mediated oxidative stress in serum of female epileptics: a possible pathogenesis in the fetal hydantoin syndrome. Hum Exp Toxicol 16:177–181
Liu Y, Gao F, Li X (2012) The anticonvulsant and neuroprotective effects of baicalin on pilocarpine-induced epileptic model in rats. Neurochem Res 37:1670–1680
Manuela M, Gaurav K, Chiara R, Rafal MK (2012) Rapid epileptogenesis in the mouse pilocarpine model: video-EEG, pharmacokinetic and histopathological characterization. Exp Neurol 238:156–167
Marchi N, Fan Q, Ghosh C, Fazio V, Bertolini F, Beto G (2009) Pilocarpine-induced seizures revisited: what does the model mimic? Epilepsy Curr 9:146–148
Mikati M, Abi-Habib R, El Sabban M, Dbaibo G, Kurdi R, Kobeissi M, Farhat F, Asaad W (2003) Hippocampal programmed cell death after status epilepticus: evidence for NMDA-receptor and ceramide-mediated mechanisms. Epilepsia 44:282–291
Pazdernik T, Emerson M, Cross R, Nelson S, Samson F (2001) Soman-induced seizures: limbic activity, oxidative stress and neuroprotective proteins. J Appl Toxicol 21:S87–S94
Peng Z, Wang S, Chen G, Cai M, Liu R, Deng J, Liu J, Zhang T, Tan Q, Hai C (2015) Gastrodin alleviates cerebral ischemic damage in mice by improving anti-oxidant and anti-inflammation activities and inhibiting apoptosis pathway. Neurochem Res 40:661–673
Perucca E, French J, Bialer M (2007) Development of new antiepileptic drugs: challenges, incentives, and recent advances. Lancet Neurol 6:793–804
Racine R (1972) Modification of seizure activity by electrical stimulation. II. Motor seizure. Electroencephalogr Clin Neurophysiol 32:281–294
Rowley S, Patel M (2013) Mitochondrial involvement and oxidative stress in temporal lobe epilepsy. Free Radic Biol Med 62:121–131
Santos L, Freitas R, Xavier S, Saldanha G, Freitas R (2008) Neuroprotective actions of vitamin C related to decreased lipid peroxidation and increased catalase activity in adult rats after pilocarpine-induced seizures. Pharmacol Biochem Behav 89:1–5
Schmitz B (2006) Effects of antiepileptic drugs on mood and behavior. Epilepsia 47:28–33
Shieh D, Liu LT, Lin CC (2000) Antioxidant and free radical scavenging effects of baicalein, baicalin and wogonin. Anticancer Res 20:2861–2865
Shin E, Jeong J, Chung Y (2011) Role of oxidative stress in epileptic seizures. Neurochem Int 59:122–137
Sudha K, Rao A, Rao A (2001) Oxidative stress and antioxidants in epilepsy. Clin Chim Acta 303:19–24
Ullah I, Badshah H, Naseer MI, Lee HY, Kim MO (2015) Thymoquinone and vitamin C attenuates pentylenetetrazole-induced seizures via activation of GABAB1 receptor in adult rats cortex and hippocampus. Neuromol Med 17:35–46
Voutsinos-Porche B, Koning E, Kaplan H, Ferrandon A, Guenounou M, Nehlig A, Motte J (2004) Temporal patterns of the cerebral inflammatory response in the rat lithium-pilocarpine model of temporal lobe epilepsy. Neurobiol Dis 17:385–402
Wang S, Chen F, Yao C (2000) The pharmacokinetic studies of alkaloids in bitter beans Chinese trad herbal. Drugs 31:1–2
Weise J, Engelhorn T, Dorfler A, Aker S, Bahr M, Hufnagel A (2005) Expression time course and spatial distribution of activated caspases-3 after experimental status epilepticus: contribution of delayed neuronal cell death to seizureinduced neuronal injury. Neurobiol Dis 18:582–590
Xie N, Wang C, Lian Y, Wu C, Zhang H, Zhang Q (2014) Puerarin protects hippocampal neurons against cell death in pilocarpine-induced seizures through antioxidant and anti-apoptotic mechanisms. Cell Mol Neurobiol 34:1175–1182. doi:10.1007/s10571-014-0093-2
Xu T, Li Y, Wang H, Xu Y, Ma L, Sun T, Ma H, Yu J (2013) Oxysophocarpine induces anti-nociception and increases the expression of GABAAα1 receptors in mice. Mol Med Rep 7(6):1819–1825
Yang Q, Zhang H, Han C (2006) Separation technology and toxicity of oxysophocarpine. J Northwest For Univ 21:111–112
Yang Y, Li Y-X, Wang H-L, Jin S-J, Zhou R, Qiao H-Q, Du J, Wu J, Zhao C-J, Niu Y, Sun T, Yu J-Q (2015) Oxysophocarpine ameliorates carrageenan-induced inflammatory pain via inhibiting expressions of prostaglandin E2 and cytokines in mice. Planta Med 81:791–797
Zeng LH, Xu L, Rensing NR, Sinatra PM, Rothman SM, Wong M (2007) Kainate seizures cause acute dendritic injury and actin depolymerization in vivo. J Neurosci 27:11604–11613
Zhao C, Li Z (2009) Modern pharmacological research of matrine alkaloid. Res Appl Vet Drugs 28:50–52
Zhao P, Zhou R, Zhu X, Hao Y, Li N (2015) Matrine attenuates focal cerebral ischemic injury by improving antioxidant activity and inhibiting apoptosis in mice. Int J Mol Med 36:633–644
Zheng H, Tang R, Yao Y, Ji Z, Cao Y, Liu Z, Peng F, Wang W, Can D, Xing H, Bu G, Xu H, Zhang YW, Zheng W (2016) MiR-219 protects against seizure in the kainic acid model of epilepsy. Mol Neurobiol 53:1–7
Zhu Q, Li Y, Zhou R, Ma N, Chang R, Wang T, Zhang Y, Chen X, Hao Y, Jin S, Ma L, Du J, Sun T, Yu J (2014) Neuroprotective effects of oxysophocarpine on neonatal rat primary cultured hippocampal neurons injured by oxygen-glucose deprivation and reperfusion. Pharm Biol Early Online:1–8
Acknowledgments
This study was supported by the Ningxia Hui Autonomous Region Science and Technology Support Program (2015BAK45B01) and the Ningxia medical university scientific research project (XY201409 and XY201514).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors have no conflicts of interest to declare.
Additional information
Gang Liu and Jing Wang authors have contributed equally to this work.
Rights and permissions
About this article
Cite this article
Liu, G., Wang, J., Deng, XH. et al. The Anticonvulsant and Neuroprotective Effects of Oxysophocarpine on Pilocarpine-Induced Convulsions in Adult Male Mice. Cell Mol Neurobiol 37, 339–349 (2017). https://doi.org/10.1007/s10571-016-0411-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10571-016-0411-y