Abstract
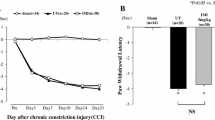
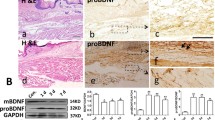
Neurotropin (NTP)®, a non-protein extract isolated from the inflamed skin of rabbits inoculated with vaccinia virus, is used clinically for the treatment of neuropathic pain. Moreover, NTP may activate the descending pain inhibitory system. Depression-like behavior is often complicated by chronic pain. However, little is known about NTP-mediated prevention of mood disorders in chronic pain and its molecular mechanisms. We aimed to investigate the effects of NTP on brain-derived neurotrophic factor (BDNF)-mediated signaling and gene expression in chronic pain. In addition, these effects of NTP were compared with pregabalin which is an anticonvulsant, anxiolytic analgesic used to treat neuropathic pain and fibromyalgia. A chronic constriction injury model was established in Sprague-Dawley rats. The pain response was assessed using a paw withdrawal latency (PWL) test and depression was assessed by the immobility time in a forced swim test (FST). NTP was orally administered in two doses of 50 NU (Neurotropin Unit) and 100 NU/kg for 7 days from day 7 after injury. To measure the analgesic and anti-depressant effects of NTP, either K252a (a tyrosine kinase inhibitor), or 5,7-dihydroxy tryptamine (5,7-DHT, a selective toxin for 5-HTergic neurons) was administered by intracerebroventricular injection. Changes in pERK1/2 and pCREB (immunohistochemistry), 5-HT, and BDNF protein level (ELISA) and BDNF mRNA (RT-PCR) were measured in the anterior cingulate cortex (ACC) and in the rostral ventromedial medulla (RVM) 14 days after injury. After injury, the rats showed a decrease in PWL associated with the increase in time of immobility in FST. In this injury model, NTP blocked both the decrease in PWL and the increase in the FST, while pregabalin (10 mg/kg, po.) did not affect the increase in the FST. These effects of NTP were reversed by K252a, and 5,7-DHT. The analgesic effects of pregabalin were not reversed by K252a. NTP normalized the injury-induced excessive activation of pERK1/2 associated with decreased pCREB and BDNF mRNA in the ACC and in the RVM, and these changes were reversed by 5,7-DHT. In contrast, pregabalin did not affect either pCREB or BDNF levels in the chronic pain model. NTP ameliorated chronic pain and pain-related depression by normalizing the induction of BDNF associated with the 5-HTergic system. Pregabalin showed the analgesic effects but had no effects on either depression or the BDNF pathway. These results suggest that NTP may represent an additional drug strategy for chronic pain associated with depression.









Similar content being viewed by others
References
Alkon DL, Epstein H, Kuzirian A, Bennett MC, Nelson TJ (2005) Protein synthesis required for long-term memory is induced by PKC activation on days before associative learning. Proc Natl Acad Sci USA 102:16432–16437
Beck T, Lindholm D, Castren E, Wree A (1994) BDNF protects against ischemic cell damage in rat HC. J Cereb Blood Flow Metab 14:689–692
Bennett GJ, Xie YK (1988) A peripheral mononeuropathy in rat that produces disorders of pain sensation like those seen in man. Pain 33:87–107
Blendy JA (2006) The role of CREB in depression and antidepressant treatment. Biol Psychiatry 59(12):1144–1150
Bremner JD, Narayan M, Anderson ER, Staib LH, Miller HL, Charney DS (2000) Hippocampal volume reduction in major depression. Am J Psychiatry 157(1):115–118
Cao H, Cui YH, Zhao ZQ, Cao XH, Zhang YQ (2009) Activation of ERK in the ACC contributes to the induction of long-term potentiation in rats. Neurosci Bull 25(5):301–308
Cao H, Ren WH, Zhu MY, Zhao ZQ, Zhang YQ (2012) Activation of glycine site and GluN2B subunit of NMDA receptors is necessary for ERK/CREB signaling cascade in rostral anterior cingulate cortex in rats: implications for affective pain. Neurosci Bull 28(1):77–87
Cejas PJ, Martinez M, Karmally S, McKillop M, McKillop J, Plunkett JA, Oudega M, Eaton MJ (2000) Lumber transplant of neurons genetically modified to secrete BDNF attenuates allodynia and hyperalgesia after sciatic nerve constriction. Pain 86:195–210
D’Sa C, Duman RS (2002) Antidepressants and neuroplasticity. Bipolar Disord 4:183-194
Duric V, McCarson KE (2005) Hippocampal neurokinin-1 receptor and BDNF gene expression is decreased in rat models of pain and stress. Neuroscience 133(4):999–1006
Fukuhara K, Ishikawa K, Yasuda S, Kishishita Y, Kim HK, Kakeda T, Yamamoto M, Norii T, Ishikawa T (2012) Intracerebroventricular 4-methylcatechol (4-MC) ameliorates chronic pain associated with depression-like behavior via induction of BDNF. Cell Mol Neurobiol 32:971–977
Fukumitsu H, Sometani A, Ohmiya M, Nitta A, Nomoto H, Furukawa Y, Furukawa S (1999) Induction of a physiologically active brain-derived neurotrophic factor in the infant rat brain by peripheral administration of 4-methylcatechol. Neurosci Lett 274:115–118
Fumagalli F, Molteni R, Calabrese F, Frasca A, Racagni G, Riva MA (2005) Chronic fluoxetine administration inhibits extracellular signal-regulated kinase 1/2 phosphorylation in rat brain. J Neurochem 93(6):1551–1560
Furukawa Y, Tomioka N, Sato W, Satoyoshi E, Hayashi K, Furukawa S (1989) Catecholamine increase nerve growth factor mRNA content in both mouse astroglial cells and fibroblast cells. FEBS Lett 247:463–467
Gao YJ, Ji RR (2009) c-Fos and pERK, which is a better marker for neuronal activation and central sensitization after noxious stimulation and tissue injury? Open Pain J 1(2):11–17
Gao YJ, Ren WH, Zhang YQ, Zhao ZQ (2004) Contributions of the ACC and amygdala to pain- and fear-conditioned place avoidance in rats. Pain 110(1–2):343–353
Gebhart GF (2004) Descending modulation of pain. Neurosci Biobehav Rev 27:729–737
Gustin SM, Wrigley PJ, Henderson LA, Siddall PJ (2010) Brain circuitry underlying pain in response to imagined movement in people with spinal cord injury. Pain 148(3):438–445
Hanaoka Y, Ohi T, Furukawa S, Furukawa Y, Hayashi K, Matsukura S (1994) The therapeutic effects of 4-methylcatechol, a stimulator of endogenous nerve growth factor synthesis, on experimental diabetic neuropathy in rats. J Neurol Sci 122:28–32
Heldt SA, Stanek L, Chhatwal JP, Ressler KJ (2007) HC-specific deletion of BDNF in adult mice impairs spatial memory and extinction of aversive memories. Mol Psychiatry 12(7):656–670
Ishikawa K (2010) Possible involvement of brain-derived neurotrophic factor in analgesic effects of 4-methylcatechol on neuropathic pain. Bull Yamaguchi Med Sch 57(3–4):49–55
Ishikawa K, Yasuda S, Fukuhara K, Iwanaga Y, Ida Y, Ishikawa J, Yamagata H, Ono M, Kakeda T, Ishikawa T (2014) 4-Methylcatechol prevents derangements of brain-derived neurotrophic factor and TrkB-related signaling in anterior cingulate cortex in chronic pain with depression-like behavior. Neuroreport 25(4):226–232
Kafitz KW, Rose CR, Thoenen H, Konnerth A (2005) Neurotrophin-evoked rapid excitation through TrkB receptors. Nature 401(6756):918–921
Kawamura M, Ohara H, Go K, Koga Y, Ienaga K (1998) Neurotropin induces antinociceptive effect by enhancing descending pain inhibitory systems involving 5-HT3 and noradrenergic alpha2 receptors in spinal dorsal horn. Life Sci 62(24):2181–2190
Kenshalo DR Jr, Giesler GJ Jr, Leonard RB, Willis WD (1980) Responses of neurons in primate ventral posterior lateral nucleus to noxious stimuli. J Neurophysiol 43:1594–1614
Lipp J (1991) Possible mechanisms of morphine analgesia. Clin Neuropharm 14:131–147
Merighi A, Salio C, Ghirri A, Lossi L, Ferrini F, Betelli C, Bardoni R (2008) BDNF as a pain modulator. Prog Neurobiol 85:297–317
Millan MJ (2002) Descending control of pain. Prog Neurobiol 66:355–474
Miura T, Okazaki R, Yoshida H, Namba H, Okai H, Kawamura M (2005) Mechanisms of analgesic action of neurotropin on chronic pain in adjuvant-induced arthritic rat: roles of descending noradrenergic and serotonergic systems. J Pharmacol Sci 97(3):429–436
Moisset X, Bouhassira D (2007) Brain imaging of neuropathic pain. Neuroimage 37:S80–88
Moisset X, Bouhassira D, Denis D, Dominique G, Benoit C, Sabaté JM (2010) Anatomical connections between brain areas activated during rectal distension in healthy volunteers: a visceral pain network. Eur J Pain 14(2):142–148
Murphy GM Jr, Sarginson JE, Ryan HS, O’Hara R, Schatzberg AF, Lazzeroni LC (2013) BDNF and CREB1 genetic variants interact to affect antidepressant treatment outcomes in geriatric depression. Pharmacogenet Genomics 23(6):301–313
Nagata K, Imai T, Yamashita T, Tsuda M, Tozaki-Saitoh H, Inoue K (2009) Antidepressants inhibit P2X4 receptor function: a possible involvement in neuropathic pain relief. Mol Pain 5:20
Okazaki R, Namba H, Yoshida H, Okai H, Miura T, Kawamura M (2008) The antiallodynic effect of Neurotropin is mediated via activation of descending pain inhibitory systems in rats with spinal nerve ligation. Anesth Analg 107(3):1064–1069
Ossipov MH, Gregory O (2010) Central modulation of pain. J Clin Invest 120:3779–3787
Paxinos G, Watson C (1998) The rat brain in stereotaxic coordinates, 4th ed. Academic Press, New York
Porsolt RD, Anton G, Blavet N, Jalfre M (1978) Behavioural despair in rats: a new model sensitive to antidepressant treatments. Euro J Pharmacol 47:379–391
Saita K, Ohi T, Hanaoka Y, Furukawa S, Furukawa Y, Hayashi K, Matsukura S (1996) A catechol derivative (4-methylcatechol) accelerates the recovery from experimental acrylamide-induced neuropathy. J Pharmacol Exp Ther 276:231–237
Schwenkreis P, Maier C, Tegenthoff M (2009) Functional imaging of central nervous system involvement in complex regional pain syndrome. AJNR Am J Neuroradiol 30(7):1279–1284
Seifert F, Maihöfner C (2009) Central mechanisms of experimental and chronic neuropathic pain: findings from functional imaging studies. Cell Mol Life Sci 66(3):375–390
Sheline YI, Wang PW, Gado MH, Csernansky JG, Vannier MW (1996) Hippocampal atrophy in recurrent major depression. Proc Natl Acad Sci USA 93:3908–3913
Shimizu E, Hashimoto K, Okamura N, Koike K, Komatsu N, Kumakiri C, Nakazato M, Watanabe H, Shinoda N, Okada S, Iyo M (2003) Alterations of serum levels of brain-derived neurotrophic factor (BDNF) in depressed patients with or without antidepressants. Biol Psychiatry 54:70-75
Sindrup SH, Otto M, Finnerup NB, Jensen TS (2005) Antidepressants in the treatment of neuropathic pain. Basic Clin Pharmacol Toxicol 96(6):399–409
Stamford JA (1995) Descending control of pain. Br J Anaesth 75:217–227
Sun MK, Alkon DL (2008) Effects of 4-methylcatechol on spatial memory and depression. NeuroReport 19(3):355–359
Suzuki R, Rygh LJ, Dickenson AH (2004) Bad news from the brain: descending 5-HT pathways that control spinal pain processing. Trends Pharmacol Sci 25:613–617
Svensson CI, Marsala M, Westerlund A, Calcutt NA, Campana WM, Freshwater JD, Catalano R, Feng Y, Protter AA, Scott B, Yaksh TL (2003) Activation of p38 mitogen-activated protein kinase in spinal microglia is a critical link in inflammation-induced spinal pain processing. J Neurochem 86:1534–1544
Tripp A, Oh H, Guilloux JP, Martinowich K, Lewis DA, Sibille E (2012) Brain-derived neurotrophic factor signaling and subgenual anterior cingulate cortex dysfunction in major depressive disorder. Am J Psychiatry 169(11):1194–1202
Vanegas H, Schaible HG (2004) Descending control of persistent pain: inhibitory or facilitatory? Brain Res Rev 46:295–309
Zeng Q, Wang S, Lim G, Yang L, Mao J, Sung B, Chang Y, Lim JA, Guo G, Mao J (2008) Exacerbated mechanical allodynia in rats with depression-like behavior. Brain Res 1200:27–38
Zhang FE, Cao JL, Zhang LC, Zeng YM (2005) Activation of p38 mitogen-activated protein kinase in spinal cord contributes to CCI-induced neuropathic pain. Pain 57(5):545–551
Conflict of interest
The authors confirm that this article content has part of conflicts of interest from Nippon Zoki Co., Ltd, Japan. (To Ishikawa T).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ishikawa, T., Yasuda, S., Minoda, S. et al. Neurotropin® Ameliorates Chronic Pain via Induction of Brain-Derived Neurotrophic Factor. Cell Mol Neurobiol 35, 231–241 (2015). https://doi.org/10.1007/s10571-014-0118-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10571-014-0118-x