Abstract
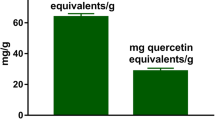
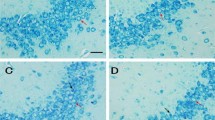
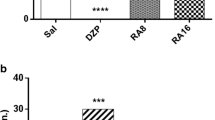
Puerarin extracted from Radix puerariae has been shown to exert neuroprotective effects. However, it is still not known whether puerarin protects hippocampal neurons against cell death in pilocarpine-induced seizures. In this study, we found that pretreatment with puerarin significantly attenuated the neuronal death in the hippocampus of rats with pilocarpine-induced epilepsy. In addition, puerarin decreased the level of seizure-induced reactive oxygen species in mitochondria isolated from the rat hippocampi. Terminal deoxyuridine triphosphate nick-end labeling staining showed that puerarin exerted an anti-apoptotic effect on the neurons in the epileptic hippocampus. Western blot analysis showed that puerarin treatment significantly decreased the expression of Bax and increased the expression of Bcl-2. Moreover, puerarin treatment restored the altered mitochondrial membrane potential and cytochrome c release from the mitochondria in the epileptic hippocampi. Altogether, the findings of this study suggest that puerarin exerts a therapeutic effect on epilepsy-induced brain injury through antioxidant and anti-apoptotic mechanisms.






Similar content being viewed by others
References
Acharya MM, Katyare SS (2005) Structural and functional alterations in mitochondrial membrane in picrotoxin-induced epileptic rat brain. Exp Neurol 192(1):79–88. doi:10.1016/j.expneurol.2004.11.004
Chang Y, Hsieh CY, Peng ZA, Yen TL, Hsiao G, Chou DS, Chen CM, Sheu JR (2009) Neuroprotective mechanisms of puerarin in middle cerebral artery occlusion-induced brain infarction in rats. J Biomed Sci 16:9. doi:10.1186/1423-0127-16-91423-0127-16-9
Chuang YC, Chang AY, Lin JW, Hsu SP, Chan SH (2004) Mitochondrial dysfunction and ultrastructural damage in the hippocampus during kainic acid-induced status epilepticus in the rat. Epilepsia 45(10):1202–1209. doi:10.1111/j.0013-9580.2004.18204.x
Fiskum G, Murphy AN, Beal MF (1999) Mitochondria in neurodegeneration: acute ischemia and chronic neurodegenerative diseases. J Cereb Blood Flow Metab 19(4):351–369. doi:10.1097/00004647-199904000-00001
Fiskum G, Rosenthal RE, Vereczki V, Martin E, Hoffman GE, Chinopoulos C, Kowaltowski A (2004) Protection against ischemic brain injury by inhibition of mitochondrial oxidative stress. J Bioenerg Biomembr 36(4):347–352. doi:10.1023/B:JOBB.0000041766.71376.81490853
Folbergrova J (2013) Oxidative stress in immature brain following experimentally-induced seizures. Physiol Res 62(Suppl 1):S39–S48
Han RM, Tian YX, Becker EM, Andersen ML, Zhang JP, Skibsted LH (2007) Puerarin and conjugate bases as radical scavengers and antioxidants: molecular mechanism and synergism with beta-carotene. J Agric Food Chem 55(6):2384–2391. doi:10.1021/jf062796c
Hui L, Pei DS, Guan QH, Zhang GY (2005) The neuroprotection of insulin on ischemic brain injury in rat hippocampus through negative regulation of JNK signaling pathway by PI3K/Akt activation. Brain Res 1052(1):1–9. doi:10.1016/j.brainres.2005.05.043
Khurana DS, Valencia I, Goldenthal MJ, Legido A (2013) Mitochondrial dysfunction in epilepsy. Semin Pediatr Neurol 20(3):176–187. doi:10.1016/j.spen.10.001
Kim KM, Jung DH, Jang DS, Kim YS, Kim JM, Kim HN, Surh YJ, Kim JS (2010) Puerarin suppresses AGEs-induced inflammation in mouse mesangial cells: a possible pathway through the induction of heme oxygenase-1 expression. Toxicol Appl Pharmacol 244(2):106–113. doi:10.1016/j.taap.2009.12.023
Qi J, Hong ZY, Xin H, Zhu YZ (2010) Neuroprotective effects of leonurine on ischemia/reperfusion-induced mitochondrial dysfunctions in rat cerebral cortex. Biol Pharm Bull 33(12):1958–1964
Qiu X, Cao L, Yang X, Zhao X, Liu X, Han Y, Xue Y, Jiang H, Chi Z (2013) Role of mitochondrial fission in neuronal injury in pilocarpine-induced epileptic rats. Neuroscience 245:157–165. doi:10.1016/j.neuroscience.2013.04.019
Racine RJ (1972) Modification of seizure activity by electrical stimulation II. Motor seizure. Electroencephalogr Clin Neurophysiol 32(3):281–294
Rowley S, Patel M (2013) Mitochondrial involvement and oxidative stress in temporal lobe epilepsy. Free Radic Biol Med 62:121–131. doi:10.1016/j.freeradbiomed.2013.02
Thummasorn S, Kumfu S, Chattipakorn S, Chattipakorn N (2011) Granulocyte-colony stimulating factor attenuates mitochondrial dysfunction induced by oxidative stress in cardiac mitochondria. Mitochondrion 11(3):457–466. doi:10.1016/j.mito.2011.01.008
Walker JM (1994) The bicinchoninic acid (BCA) assay for protein quantitation. Methods Mol Biol 32:5–8. doi:10.1385/0-89603-268-X:5
Xie N, Li H, Wei D, LeSage G, Chen L, Wang S, Zhang Y, Chi L, Ferslew K, He L, Chi Z, Yin D (2010a) Glycogen synthase kinase-3 and p38 MAPK are required for opioid-induced microglia apoptosis. Neuropharmacology 59(6):444–451. doi:10.1016/j.neuropharm.2010.06.006
Xie N, Wang C, Lin Y, Li H, Chen L, Zhang T, Sun Y, Zhang Y, Yin D, Chi Z (2010b) The role of p38 MAPK in valproic acid induced microglia apoptosis. Neurosci Lett 482(1):51–56. doi:10.1016/j.neulet.2010.07.004
Xu X, Zheng X (2007) Potential involvement of calcium and nitric oxide in protective effects of puerarin on oxygen-glucose deprivation in cultured hippocampal neurons. J Ethnopharmacol 113(3):421–426. doi:10.1016/j.jep.2007.06.012
Xu J, Wang S, Lin Y, Cao L, Wang R, Chi Z (2009) Ghrelin protects against cell death of hippocampal neurons in pilocarpine-induced seizures in rats. Neurosci Lett 453(1):58–61. doi:10.1016/j.neulet.2009.01.067
Acknowledgments
This study was supported by a Grant from National Natural Science Foundation of China (No. 81201012, 81371438), the Youth Innovation Fund of the First Affiliated Hospital of Zhengzhou University and China Postdoctoral Science Foundation Special Funded Project (No. 2013T60707).
Conflict of interest
The authors have no conflicts of interest to declare.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Xie, N., Wang, C., Lian, Y. et al. Puerarin Protects Hippocampal Neurons Against Cell Death in Pilocarpine-Induced Seizures Through Antioxidant and Anti-Apoptotic Mechanisms. Cell Mol Neurobiol 34, 1175–1182 (2014). https://doi.org/10.1007/s10571-014-0093-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10571-014-0093-2