Abstract
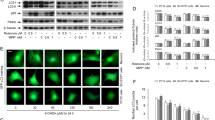
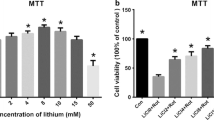
14-3-3 proteins have been confirmed to be involved in Parkinson’s disease. It has been reported that an increase of 14-3-3 (theta, epsilon, and gamma) expression has neuroprotective effect in response to rotenone and MPP+ in dopaminergic cell culture and transgenic C. elegans with alpha-synuclein overexpression. To further investigate the detail mechanism of 14-3-3 proteins in rotenone-induced dopamine neurotoxicity, we observed the expression of 14-3-3 isoforms, and the influence of 14-3-3epsilon knockdown on autophagic activity and cell function. The results showed that rotenone led to a decrease in expression of 14-3-3 protein and mRNA, and an increase in expression and aggregation of alpha-synuclein protein. Knockdown of 14-3-3epsilon expression in turn further aggravated PC12 cell damage, such as an enhancement of ROS formation, and a reduction of cell viability and ATP production. Further experiments confirmed that the autophagic activity was promoted with 14-3-3epsilon siRNA transfection, including an enhancement of autophagosome formation and the ratio of LC3-II/LC3-I. Therefore, we concluded that the regulation of 14-3-3 proteins in rotenone-induced neurotoxicity might be associated with its isoform 14-3-3epsilon’s involvement in autophagy, which might be considered a mechanism in addition to the currently known function of 14-3-3 proteins in neurodegenerative disease pathogenesis.








Similar content being viewed by others
References
Boston PF, Jackson P, Thompson RJ (1982) Human 14-3-3 protein: radioimmunoassay, tissue distribution, and cerebrospinal fluid levels in patients with neurological disorders. J Neurochem 38:1475–1482
Bustos DM (2012) The role of protein disorder in the 14-3-3 interaction network. Mol Biosyst 8:178–184
Cannon JR, Greenamyre JT (2010) Neurotoxic in vivo models of Parkinson’s disease recent advances. Prog Brain Res 184:17–33
Chaabane W, User SD, El-Gazzah M, Jaksik R, Sajjadi E, Rzeszowska-Wolny J, Los MJ (2013) Autophagy, apoptosis, mitoptosis and necrosis: interdependence between those pathways and effects on cancer. Arch Immunol Ther Exp 61:43–58
Chen XW, Wang T, Yuan KX, Wang YJ, Wang SR, Sun SG, Chen ZB (2012) Adenovirus vector mediated human 14-3-3 gamma gene transfer protects dopaminergic cells against rotenone-induced injury. Zhonghua Yi Xue Za Zhi 92:55–59
Ebrahimi-Fakhari D, Wahlster L, McLean PJ (2012) Protein degradation pathways in Parkinson’s disease: curse or blessing. Acta Neuropathol 124:153–172
Ichimura T, Isobe T, Okuyama T, Yamauchi T, Fujisawa H (1987) Brain 14-3-3 protein is an activator protein that activates tryptophan 5-monooxygenase and tyrosine 3-monooxygenase in the presence of Ca2+, calmodulin-dependent protein kinase II. FEBS Lett 219:79–82
Jafar-Nejad P, Ward CS, Richman R, Orr HT, Zoghbi HY (2011) Regional rescue of spinocerebellar ataxia type 1 phenotypes by 14-3-3epsilon haploinsufficiency in mice underscores complex pathogenicity in neurodegeneration. Proc Natl Acad Sci USA 108:2142–2147
Kawamoto Y, Akiguchi I, Nakamura S, Honjyo Y, Shibasaki H, Budka H (2002) 14-3-3 proteins in Lewy bodies in Parkinson disease and diffuse Lewy body disease brains. J Neuropathol Exp Neurol 61:245–253
Kurz A, May C, Schmidt O, Muller T, Stephan C, Meyer HE, Gispert S, Auburger G, Marcus K (2012) A53T-alpha-synuclein-overexpression in the mouse nigrostriatal pathway leads to early increase of 14-3-3epsilon and late increase of GFAP. J Neural Transm 119:297–312
Mackie S, Aitken A (2005) Novel brain 14-3-3 interacting proteins involved in neurodegenerative disease. FEBS J 272:4202–4210
Nistico R, Mehdawy B, Piccirilli S, Mercuri N (2011) Paraquat- and rotenone-induced models of Parkinson’s disease. Int J Immunopathol Pharmacol 24:313–322
Ostrerova N, Petrucelli L, Farrer M, Mehta N, Choi P, Hardy J, Wolozin B (1999) Alpha-Synuclein shares physical and functional homology with 14-3-3 proteins. J Neurosci 19:5782–5791
Pickford F, Masliah E, Britschgi M, Lucin K, Narasimhan R, Jaeger PA, Small S, Spencer B, Rockenstein E, Levine B, Wyss-Coray T (2008) The autophagy-related protein beclin 1 shows reduced expression in early Alzheimer disease and regulates amyloid beta accumulation in mice. J Clin Invest 118:2190–2199
Pozuelo-Rubio M (2011a) 14-3-3zeta binds class III phosphatidylinositol-3-kinase and inhibits autophagy. Autophagy 7:240–242
Pozuelo-Rubio M (2011b) Regulation of autophagic activity by 14-3-3zeta proteins associated with class III phosphatidylinositol-3-kinase. Cell Death Differ 18:479–492
Roseboom PH, Weller JL, Babila T, Aitken A, Sellers LA, Moffett JR, Namboodiri MA, Klein DC (1994) Cloning and characterization of the epsilon and zeta isoforms of the 14-3-3 proteins. DNA Cell Biol 13:629–640
Sai Y, Wu Q, Le W, Ye F, Li Y, Dong Z (2008) Rotenone-induced PC12 cell toxicity is caused by oxidative stress resulting from altered dopamine metabolism. Toxicol In Vitro 22:1461–1468
Satoh K, Shirabe S, Eguchi H, Tsujino A, Eguchi K, Satoh A, Tsujihata M, Niwa M, Katamine S, Kurihara S, Matsuo H (2006) 14-3-3 protein, total tau and phosphorylated tau in cerebrospinal fluid of patients with Creutzfeldt-Jakob disease and neurodegenerative disease in Japan. Cell Mol Neurobiol 26:45–52
Shirakashi Y, Kawamoto Y, Tomimoto H, Takahashi R, Ihara M (2006) Alpha-Synuclein is colocalized with 14-3-3 and synphilin-1 in A53T transgenic mice. Acta Neuropathol 112:681–689
Slone SR, Lesort M, Yacoubian TA (2011) 14-3-3theta protects against neurotoxicity in a cellular Parkinson’s disease model through inhibition of the apoptotic factor Bax. PLoS One 6:e21720
Son JH, Shim JH, Kim KH, Ha JY, Han JY (2012) Neuronal autophagy and neurodegenerative diseases. Exp Mol Med 44:89–98
Steinacker P, Aitken A, Otto M (2011) 14-3-3 proteins in neurodegeneration. Semin Cell Dev Biol 22:696–704
Umahara T, Uchihara T, Nakamura A, Iwamoto T (2011) Differential expression of 14-3-3 protein isoforms in developing rat hippocampus, cortex, rostral migratory stream, olfactory bulb, and white matter. Brain Res 1410:1–11
Wang B, Ling S, Lin WC (2010) 14-3-3Tau regulates Beclin 1 and is required for autophagy. PLoS One 5:e10409
Wang RC, Wei Y, An Z, Zou Z, Xiao G, Bhagat G, White M, Reichelt J, Levine B (2012) Akt-mediated regulation of autophagy and tumorigenesis through Beclin 1 phosphorylation. Science 338:956–959
Xu J, Kao SY, Lee FJ, Song W, Jin LW, Yankner BA (2002) Dopamine-dependent neurotoxicity of alpha-synuclein: a mechanism for selective neurodegeneration in Parkinson disease. Nat Med 8:600–606
Yacoubian T, Slone S, Harrington A, Hamamichi S, Schieltz J, Caldwell K, Caldwell G, Standaert D (2010) Differential neuroprotective effects of 14-3-3 proteins in models of Parkinson’s disease. Cell Death Dis 1:e2
Acknowledgments
This work was supported by grants from NSFC (Natural Science Foundation of China) (30800932, 81273106) to Yan Sai and Natural Science Foundation of Chongqing (cstc2012jjA10028) to Yan Sai.
Conflict of interest
The authors declare no conflict of interest.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Sai, Y., Peng, K., Ye, F. et al. 14-3-3 Proteins in the Regulation of Rotenone-Induced Neurotoxicity Might be via Its Isoform 14-3-3Epsilon’s Involvement in Autophagy. Cell Mol Neurobiol 33, 1109–1121 (2013). https://doi.org/10.1007/s10571-013-9977-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10571-013-9977-9