Abstract
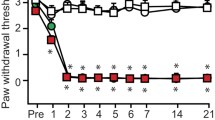
The analgesic effect induced by opiates is often potentiated during experimental inflammatory processes. We describe here that lower doses of systemic morphine are necessary to increase thermal withdrawal latencies measured in both hind paws of mice acutely inflamed with carrageenan than in healthy ones. This bilateral potentiation seems mediated through spinal opioid receptors since it is inhibited by the intrathecal (i.t.), but not intraplantar (i.pl.) administration of the opioid receptor antagonist naloxone-methiodide, and also appears when morphine is i.t. administered. Furthermore, the i.pl. administration of the nitric oxide (NO) synthase inhibitor, l-NMMA, or the K +ATP -channel blocker, glibenclamide, to carrageenan-inflamed mice inhibits the enhanced effect of systemic morphine in the paw that receives the injection of the drug, without affecting the potentiation observed in the contralateral one. The i.pl. administration of l-NMMA also partially antagonised the analgesic effect induced by i.t. morphine in inflamed mice. Finally, the increased analgesic effect evoked by the i.pl. administration of the NO donor SIN-1 either in the inflamed or in the contralateral paw of carrageenan-inflamed mice suggests that enhanced responsiveness to the peripheral analgesic effect of NO may be also underlying the bilateral potentiation of morphine-induced analgesia in acutely inflamed mice.






Similar content being viewed by others
References
Brock SC, Tonussi CR (2008) Intrathecally injected morphine inhibits inflammatory paw edema: the involvement of nitric oxide and cyclic-guanosine monophosphate. Anesth Analg 106:965–971
Curto-Reyes V, Juárez L, García-Pérez E, Fresno MF, Hidalgo A, Menéndez L, Baamonde A (2008) Local loperamide inhibits thermal hyperalgesia but not mechanical allodynia induced by intratibial inoculation of melanoma cells in mice. Cell Mol Neurobiol 28:981–990
Fecho K, Manning EL, Maixner W, Schmitt CP (2007) Effects of carrageenan and morphine on acute inflammation and pain in Lewis and Fischer rats. Brain Behav Immun 21:68–78
Ferreira SH, Lorenzetti BB (1994) Glutamate spinal retrograde sensitization of primary sensory neurons associated with nociception. Neuropharmacology 33:1479–1485
Ferreira SH, Duarte ID, Lorenzetti BB (1991) The molecular mechanism of action of peripheral morphine analgesia: stimulation of the cGMP system via nitric oxide release. Eur J Pharmacol 201:121–122
Funez MI, Ferrari LF, Duarte DB, Sachs D, Cunha FQ, Lorenzetti BB, Parada CA, Ferreira SH (2008) Inaugural article: teleantagonism: a pharmacodynamic property of the primary nociceptive neuron. Proc Natl Acad Sci USA 105:19038–19043
Hassan AH, Ableitner A, Stein C, Herz A (1993) Inflammation of the rat paw enhances axonal transport of opioid receptors in the sciatic nerve and increases their density in the inflamed tissue. Neuroscience 55:185–195
Hurley RW, Hammond DL (2000) The analgesic effects of supraspinal mu and delta opioid receptor agonists are potentiated during persistent inflammation. J Neurosci 20:1249–1259
Hylden JL, Wilcox GL (1980) Intrathecal morphine in mice: a new technique. Eur J Pharmacol 17:313–316
Hylden JL, Thomas DA, Iadarola MJ, Nahin RL, Dubner R (1991) Spinal opioid analgesic effects are enhanced in a model of unilateral inflammation/hyperalgesia: possible involvement of noradrenergic mechanisms. Eur J Pharmacol 194:135–143
Joris J, Costello A, Dubner R, Hargreaves KM (1990) Opiates suppress carrageenan-induced edema and hyperthermia at doses that inhibit hyperalgesia. Pain 43:95–103
Kayser V, Guilbaud G (1983) The analgesic effects of morphine, but not those of the enkephalinase inhibitor thiorphan, are enhanced in arthritic rats. Brain Res 267:131–138
Luger NM, Sabino MA, Schwei MJ, Mach DB, Pomonis JD, Keyser CP, Rathbun M, Clohisy DR, Honore P, Yaksh TL, Mantyh PW (2002) Efficacy of systemic morphine suggests a fundamental difference in the mechanisms that generate bone cancer vs inflammatory pain. Pain 99:397–406
Marker CL, Stoffel M, Wickman K (2004) Spinal G-protein-gated K+ channels formed by GIRK1 and GIRK2 subunits modulate thermal nociception and contribute to morphine analgesia. J Neurosci 24:2806–2812
Menéndez L, Lastra A, Hidalgo A, Baamonde A (2002) Unilateral hot plate test: a simple and sensitive method for detecting central and peripheral hyperalgesia in mice. J Neurosci Methods 113:91–97
Menéndez L, Juárez L, García V, Hidalgo A, Baamonde A (2007) Involvement of nitric oxide in the inhibition of bone cancer-induced hyperalgesia through the activation of peripheral opioid receptors in mice. Neuropharmacology 53:71–80
Millan MJ, Członkowski A, Pilcher CW, Almeida OF, Millan MH, Colpaert FC, Herz A (1987) A model of chronic pain in the rat: functional correlates of alterations in the activity of opioid systems. J Neurosci 7:77–87
Millan MJ, Członkowski A, Morris B, Stein C, Arendt R, Huber A, Höllt V, Herz A (1988) Inflammation of the hind limb as a model of unilateral, localized pain: influence on multiple opioid systems in the spinal cord of the rat. Pain 35:299–312
Parada CA, Vivancos GG, Tambeli CH, Cunha FQ, Ferreira SH (2003) Activation of presynaptic NMDA receptors coupled to NaV1.8-resistant sodium channel C-fibers causes retrograde mechanical nociceptor sensitization. Proc Natl Acad Sci USA 100:2923–2928
Perrot S, Guilbaud G, Kayser V (2001) Differential behavioral effects of peripheral and systemic morphine and naloxone in a rat model of repeated acute inflammation. Anesthesiology 94:870–875
Przewłocka B, Dziedzicka M, Lasoń W, Przewłocki R (1992) Differential effects of opioid receptor agonists on nociception and cAMP level in the spinal cord of monoarthritic rats. Life Sci 50:45–54
Przewlocki R, Przewlocka B (2005) Opioids in neuropathic pain. Curr Pharm Des 11:3013–3025
Przewłocki R, Przewłocka B (2001) Opioids in chronic pain. Eur J Pharmacol 429:79–91
Sachs D, Cunha FQ, Ferreira SH (2004) Peripheral analgesic blockade of hypernociception: activation of arginine/NO/cGMP/protein kinase G/ATP-sensitive K+ channel pathway. Proc Natl Acad Sci USA 101:3680–3685
Shaqura MA, Zöllner C, Mousa SA, Stein C, Schäfer M (2004) Characterization of mu opioid receptor binding and G protein coupling in rat hypothalamus, spinal cord, and primary afferent neurons during inflammatory pain. J Pharmacol Exp Ther 308:712–718
Spataro LE, Sloane EM, Milligan ED, Wieseler-Frank J, Schoeniger D, Jekich BM, Barrientos RM, Maier SF, Watkins LR (2004) Spinal gap junctions: potential involvement in pain facilitation. J Pain 5:392–405
Stanfa LC, Sullivan AF, Dickenson AH (1992) Alterations in neuronal excitability and the potency of spinal mu, delta and kappa opioids after carrageenan-induced inflammation. Pain 50:345–354
Sykes KT, White SR, Hurley RW, Mizoguchi H, Tseng LF, Hammond DL (2007) Mechanisms responsible for the enhanced antinociceptive effects of micro-opioid receptor agonists in the rostral ventromedial medulla of male rats with persistent inflammatory pain. J Pharmacol Exp Ther 322:813–821
Vivancos GG, Parada CA, Ferreira SH (2003) Opposite nociceptive effects of the arginine/NO/cGMP pathway stimulation in dermal and subcutaneous tissues. Br J Pharmacol 138:1351–1357
Whiteside GT, Boulet JM, Walker K (2005) The role of central and peripheral mu opioid receptors in inflammatory pain and edema: a study using morphine and DiPOA ([8-(3, 3-diphenyl-propyl)-4-oxo-1-phenyl-1, 3, 8-triaza-spiro[4.5]dec-3-yl]-acetic acid). J Pharmacol Exp Ther 314:1234–1240
Acknowledgments
Grants were provided by MEC-FEDER (SAF2006-05226). The Instituto Universitario de Oncología is supported by Obra Social Cajastur-Asturias, Spain.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
González-Rodríguez, S., Hidalgo, A., Baamonde, A. et al. Spinal and Peripheral Mechanisms Involved in the Enhancement of Morphine Analgesia in Acutely Inflamed Mice. Cell Mol Neurobiol 30, 113–121 (2010). https://doi.org/10.1007/s10571-009-9436-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10571-009-9436-9