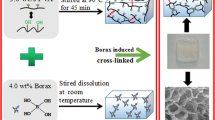
Abstract
Gas phase coagulation is a highly efficient method for fabricating physically crosslinked nanochitin-based hydrogels. In this study, a visible and tailorable strategy was developed using phenolphthalein and litmus indicator. The indicator was applied to stain partially deacetylated nanochitin (DEChNs), ensuring that the gelation process occurring during gas phase coagulation was able to be clearly visualized. A semi-gelatinized interface cross-sectional view was obtained, leading to the possibility to monitor and optimize the crosslinking process. Thereafter, a series of optimizations was performed to improve the nanochitin gelation process. Only 16.5 h was required for sufficient physical crosslinking when the molar ratio of ammonia to acetic acid was 3.45. In mixtures containing this proportion, the ammonia solution was completely reduced and the formation process was able to be controlled and improved. A higher concentration of nanochitin led to a slower cross-linking process, but increased the mechanical strength and produced a more uniform structure, which might be due to the formation of a more uniform network at an appropriate gelation rate. The optimization of the physical cross-linking process will improve our understanding of the mechanisms underlying the gas phase coagulation technique and the control of the properties of nanochitin hydrogels and their composites by altering the cross-linking conditions.
Graphic abstract
The nanochitin gelation process was clearly visualized and optimized with substantially reduces the amount of ammonia solution and the precisely predicts and controls time, while it maintains a homogeneous and stable network structure, a higher specific surface and the same strong mechanical properties of the hydrogel.













Similar content being viewed by others
References
Abe K, Ifuku S, Kawata M, Yano H (2014) Preparation of tough hydrogels based on β-chitin nanofibers via NaOH treatment. Cellulose 21:535–540
Akhtar MF, Hanif M, Ranjha NM (2016) Methods of synthesis of hydrogels … a review. Saudi Pharm J 24:554–559
Argüelles-Monal W, Goycoolea FM, Peniche C, Higuera-Ciapara I (1998) Rheological study of the chitosan/glutaraldehyde chemical gel system. Polym Gels Netw 6:429–440
Busilacchi A, Gigante A, Mattioli-Belmonte M et al (2013) Chitosan stabilizes platelet growth factors and modulates stem cell differentiation toward tissue regeneration. Carbohydr Polym 98:665–676
Cai J, Zhang L (2006) Unique gelation behavior of cellulose in NaOH/urea aqueous solution. Biomacromolecules 7:183–189
Ding B, Cai J, Huang J et al (2012) Facile preparation of robust and biocompatible chitin aerogels. J Mater Chem 22:5801
Fan Y, Saito T, Isogai A (2008) Preparation of chitin nanofibers from squid pen β-chitin by simple mechanical treatment under acid conditions. Biomacromolecules 9:1919–1923
Hennink WE, van Nostrum CF (2012) Novel crosslinking methods to design hydrogels. Adv Drug Deliv Rev 64:223–236
Hu X, Tang Y, Wang Q et al (2011) Rheological behaviour of chitin in NaOH/urea aqueous solution. Carbohydr Polym 83:1128–1133
Job N, Théry A, Pirard R et al (2005) Carbon aerogels, cryogels and xerogels: influence of the drying method on the textural properties of porous carbon materials. Carbon N Y 43:2481–2494
Khor E, Lim LY (2003) Implantable applications of chitin and chitosan. Biomaterials 24:2339–2349
Kopeček J (2007) Hydrogel biomaterials: a smart future? Biomaterials 28:5185–5192
Ling S, Chen W, Fan Y et al (2018) Biopolymer nanofibrils: structure, modeling, preparation, and applications. Prog Polym Sci 85:1–56
Liu L, Wang R, Yu J et al (2016) Robust self-standing chitin nanofiber/nanowhisker hydrogels with designed surface charges and ultralow mass content via gas phase coagulation. Biomacromolecules 17:3773–3781
Min B-M, Lee SW, Lim JN et al (2004) Chitin and chitosan nanofibers: electrospinning of chitin and deacetylation of chitin nanofibers. Polymer (Guildf) 45:7137–7142
Mushi NE, Kochumalayil J, Cervin NT et al (2016) Nanostructurally controlled hydrogel based on small-diameter native chitin nanofibers: preparation, structure, and properties. ChemSusChem 9:989–995
Muzzarelli RAA, Muzzarelli C (2009) Chitin and chitosan hydrogels. In: Phillips GO, Williams PA (eds) Handbook of hydrocolloids. CRC Press, Boca Raton, pp 849–888
Peppas N (2000) Hydrogels in pharmaceutical formulations. Eur J Pharm Biopharm 50:27–46
Rinaudo M (2006) Chitin and chitosan: properties and applications. Prog Polym Sci 31:603–632
Rwei S-P, Lyu M-S, Wu P-S et al (2009) Sol/gel transition and liquid crystal transition of HPC in ionic liquid. Cellulose 16:9–17
Sannino A, Madaghiele M, Lionetto MG et al (2006) A cellulose-based hydrogel as a potential bulking agent for hypocaloric diets: an in vitro biocompatibility study on rat intestine. J Appl Polym Sci 102:1524–1530
Shu X, Zhu K (2002) Controlled drug release properties of ionically cross-linked chitosan beads: the influence of anion structure. Int J Pharm 233:217–225
Van Vlierberghe S, Dubruel P, Schacht E (2011) Biopolymer-based hydrogels as scaffolds for tissue engineering applications: a review. Biomacromolecules 12:1387–1408
Younes I, Rinaudo M (2015) Chitin and chitosan preparation from marine sources. Structure, properties and applications. Mar Drugs 13:1133–1174
Acknowledgments
We are grateful for the financial support from the Natural Science Foundation of Jiangsu Province (BK20190761), University of Jiangsu Province Natural Science Foundation Project (19KJB430004), Jiangsu Planned Projects for Postdoctoral Research Funds (2019K101) and a Project Funded by the National First-class Disciplines (PNFD), a Project Funded by the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ma, H., Xu, J., Yu, J. et al. Visualization and improvement of the physical gelation process during gas phase coagulation through acid–base indicator staining, monitoring and optimization. Cellulose 27, 6871–6886 (2020). https://doi.org/10.1007/s10570-020-03267-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10570-020-03267-7