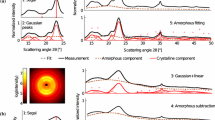
Abstract
This paper addresses two fundamental issues in the peak deconvolution method of cellulose XRD data analysis: there is no standard model for amorphous cellulose and common peak functions such as Gauss, Lorentz and Voigt functions do not fit the amorphous profile well. It first examines the effects of ball milling on three types of cellulose and results show that ball milling transforms all samples into a highly amorphous phase exhibiting nearly identical powder X-ray diffraction (XRD) profiles. It is hypothesized that short range order within a glucose unit and between adjacent units survives ball milling and generates the characteristic amorphous XRD profiles. This agrees well with cellulose I d-spacing measurements and oligosaccharide XRD analysis. The amorphous XRD profile is modeled using a Fourier series equation where the coefficients are determined using the nonlinear least squares method. A new peak deconvolution method then is proposed to analyze cellulose XRD data with the amorphous Fourier model function in conjunction with standard Voigt functions representing the crystalline peaks. The impact of background subtraction method has also been assessed. Analysis of several cellulose samples was then performed and compared to the conventional peak deconvolution methods with common peak fitting functions and background subtraction approach. Results suggest that prior peak deconvolution methods overestimate cellulose crystallinity.
Graphic abstract












Similar content being viewed by others
References
Ahvenainen P, Kontro I, Svedström K (2016) Comparison of sample crystallinity determination methods by X-ray diffraction for challenging cellulose I materials. Cellulose 23:1–14
Astley OM, Chanliaud E, Donald AM, Gidley MJ (2001) Structure of acetobacter cellulose composites in the hydrated state. Int J Biol Macromol 29:193–202. https://doi.org/10.1016/S0141-8130(01)00167-2
Azubuike CP, Rodríguez H, Okhamafe AO, Rogers RD (2012) Physicochemical properties of maize cob cellulose powders reconstituted from ionic liquid solution. Cellulose 19:425–433. https://doi.org/10.1007/s10570-011-9631-y
Bansal P (2011) Computational and experimental investigation of the enzymatic hydrolysis of cellulose. Georgia Institue of Technology, Atlanta
Bansal P, Hall M, Realff MJ et al (2010) Multivariate statistical analysis of X-ray data from cellulose: a new method to determine degree of crystallinity and predict hydrolysis rates. Bioresour Technol 101:4461–4471. https://doi.org/10.1016/j.biortech.2010.01.068
Barnette AL, Lee C, Bradley LC et al (2012) Quantification of crystalline cellulose in lignocellulosic biomass using sum frequency generation (SFG) vibration spectroscopy and comparison with other analytical methods. Carbohydr Polym 89:802–809. https://doi.org/10.1016/j.carbpol.2012.04.014
Bates S, Zografi G, Engers D et al (2006) Analysis of amorphous and nanocrystalline solids from their X-Ray diffraction patterns. Pharm Res 23:2333–2349. https://doi.org/10.1007/s11095-006-9086-2
Buerger M, Klein GE (1945) Correction of X-ray diffraction Intensities for Lorentz and Polarization Factors. J Appl Phys 16:408–418
Ciolacu D, Ciolacu F, Popa VI (2011) Amorphous cellulose—structure and characterization. Cellul Chem Technol 45:13
Cullity BD (1978) Elements of x-ray diffraction. Addison-Wesley Pub. Co., Reading
Delhez R, De Keijser TH, Mittemeijer EJ (1982) Determination of crystallite size and lattice distortions through X-ray diffraction line profile analysis. Fresenius Z Für Anal Chem 312:1–16
Delhez R, de Keijser TH, Langford JI, Louër D, Mittemeijer EJ, Sonneveld, EJ (1993) Crystal imperfection broadening and peak shape in the Rietveld method. In: Young RA (ed) The Rietveld Method. Oxford University Press, p 132
Dinnebier RE (2008) Powder diffraction: theory and practice. Royal Society of Chemistry, Cambridge
Dumitriu S (2004) Polysaccharides: structural diversity and functional versatility, 2nd edn. CRC Press, Boca Raton
Fang L, Catchmark JM (2014) Structure characterization of native cellulose during dehydration and rehydration. Cellulose 21:3951–3963. https://doi.org/10.1007/s10570-014-0435-8
Fawcett TG, Crowder CE, Kabekkodu SN et al (2013) Reference materials for the study of polymorphism and crystallinity in cellulosics. Powder Diffr 28:18–31
Fink H-P, Philipp B, Paul D et al (1987) The structure of amorphous cellulose as revealed by wide-angle X-ray scattering. Polymer 28:1265–1270. https://doi.org/10.1016/0032-3861(87)90435-6
Fourier J (1822) Theorie analytique de la chaleur, par M. Fourier. chez Firmin Didot, pere et fils
French AD (2014) Idealized powder diffraction patterns for cellulose polymorphs. Cellulose 21:885–896. https://doi.org/10.1007/s10570-013-0030-4
French AD, Santiago Cintrón M (2013) Cellulose polymorphy, crystallite size, and the Segal Crystallinity Index. Cellulose 20:583–588. https://doi.org/10.1007/s10570-012-9833-y
Garvey CJ, Parker IH, Simon GP (2005) On the interpretation of X-Ray diffraction powder patterns in terms of the nanostructure of cellulose I fibres. Macromol Chem Phys 206:1568–1575. https://doi.org/10.1002/macp.200500008
Guo J, Catchmark JM (2012) Surface area and porosity of acid hydrolyzed cellulose nanowhiskers and cellulose produced by Gluconacetobacter xylinus. Carbohydr Polym 87:1026–1037. https://doi.org/10.1016/j.carbpol.2011.07.060
He J, Cui S, Wang S (2008) Preparation and crystalline analysis of high-grade bamboo dissolving pulp for cellulose acetate. J Appl Polym Sci 107:1029–1038. https://doi.org/10.1002/app.27061
Hermans PH, Weidinger A (1946) On the diffusely diffracted radiation in the X-ray diagrams of cellulose fibres. Recl Trav Chim Pays-Bas 65:620–623. https://doi.org/10.1002/recl.19460650814
Hult E-L, Iversen T, Sugiyama J (2003) Characterization of the supermolecular structure of cellulose in wood pulp fibres. Cellulose 10:103–110. https://doi.org/10.1023/A:1024080700873
Ioelovich M, Leykin A, Figovsky O (2010) Study of cellulose paracrystallinity. BioResources 5:1393–1407. https://doi.org/10.15376/biores.5.3.1393-1407
Jmol (2016) An open-source Java viewer for chemical structures in 3D. https://jmol.sourceforge.net/. Accessed 25 June 2016
Ju X, Bowden M, Brown EE, Zhang X (2015) An improved X-ray diffraction method for cellulose crystallinity measurement. Carbohydr Polym 123:476–481. https://doi.org/10.1016/j.carbpol.2014.12.071
Klug HP, Alexander LE (1974) X-Ray Diffraction procedures: for polycrystalline and amorphous materials, 2nd edn. Wiley, New York
Kroon-Batenburg LM, Kruiskamp PH, Vliegenthart JF, Kroon J (1997) Estimation of the persistence length of polymers by MD simulations on small fragments in solution. Application to cellulose. J Phys Chem B 101:8454–8459
Langford JI, Wilson AJC (1978) Scherrer after sixty years: a survey and some new results in the determination of crystallite size. J Appl Crystallogr 11:102–113
Lanson B (1997) Decomposition of experimental X-ray diffraction patterns (profile fitting); a convenient way to study clay minerals. Clays Clay Miner 45:132–146
Ling Z, Wang T, Makarem M, Santiago Cintrón M, Cheng HN, Kang X, Bacher M, Potthast A, Rosenau T, King H, Delhom CD, Nam S, Edwards JV, Kim SH, Xu F, French AD (2019) Effects of ball milling on the structure of cotton cellulose. Cellulose 26:305–328
Luca Lutterotti MAUD (2020) Material analysis using diffraction. https://maud.radiographema.eu/. Accessed 14 Feb 2020
Madsen IC, Scarlett NVY, Kern A (2011) Description and survey of methodologies for the determination of amorphous content via X-ray powder diffraction. Z Für Krist Cryst Mater 226:944–955
McCusker LB, Von Dreele RB, Cox DE et al (1999) Rietveld refinement guidelines. J Appl Crystallogr 32:36–50
Mittemeijer EJ, Welzel U (2008) The “state of the art” of the diffraction analysis of crystallite size and lattice strain. Z Für Krist 223:552–560
Nada Stubičar IŠ (1998) An X-Ray Diffraction study of the crystalline to amorphous phase change in cellulose during high-energy dry ball milling. Holzforschung 52:455–458. https://doi.org/10.1515/hfsg.1998.52.5.455
Nelson ML, O’Connor RT (1964) Relation of certain infrared bands to cellulose crystallinity and crystal latticed type. Part I. Spectra of lattice types I, II, III and of amorphous cellulose. J Appl Polym Sci 8:1311–1324. https://doi.org/10.1002/app.1964.070080322
Park S, Baker JO, Himmel ME et al (2010) Cellulose crystallinity index: measurement techniques and their impact on interpreting cellulase performance. Biotechnol Biofuels 3:10. https://doi.org/10.1186/1754-6834-3-10
Park S, Johnson DK, Ishizawa CI et al (2009) Measuring the crystallinity index of cellulose by solid state 13C nuclear magnetic resonance. Cellulose 16:641–647. https://doi.org/10.1007/s10570-009-9321-1
Pecharsky V, Zavalij P (2008) Fundamentals of powder diffraction and structural characterization of materials, 2nd edn. Springer, Berlin
Pires L, de Figueiredo F, Ferreira F (2014) The Rietveld method as a tool to quantify the amorphous amount of microcrystalline cellulose. J Pharm Sci 103:1394. https://doi.org/10.1002/jps.23909
Rodgers JL, Nicewander WA (1988) Thirteen ways to look at the correlation coefficient. Am Stat 42:59–66. https://doi.org/10.2307/2685263
Schwanninger M, Rodrigues JC, Pereira H, Hinterstoisser B (2004) Effects of short-time vibratory ball milling on the shape of FT-IR spectra of wood and cellulose. Vib Spectrosc 36:23–40. https://doi.org/10.1016/j.vibspec.2004.02.003
Segal L, Creely JJ, Martin AE, Conrad CM (1959) An empirical method for estimating the degree of crystallinity of native cellulose using the X-Ray diffractometer. Text Res J 29:786–794. https://doi.org/10.1177/004051755902901003
Stankovic L, Dakovic M, Thayaparan T (2013) Time-frequency signal analysis with applications. Artech House Publishers, Norwood, MA
Suortti P (1993) Bragg reflection profile shape in X-ray powder diffraction patterns. In: Young RA (ed) The Rietveld method. Cambridge University Press, Cambridge, pp 167–185
Swenson HA, Schmitt CA, Thompson NS (1965) Comparison of the configuration of β-1,4 linked hexosans and wood xylan pentosans by means of the eizner-ptitsyn viscosity equations. J Polym Sci Part C Polym Symp 11:243–252. https://doi.org/10.1002/polc.5070110117
Taylor LS, Zografi G (1998) The quantitative analysis of crystallinity using FT-Raman spectroscopy. Pharm Res 15:755–761. https://doi.org/10.1023/A:1011979221685
Teeäär R, Serimaa R, Paakkarl T (1987) Crystallinity of cellulose, as determined by CP/MAS NMR and XRD methods. Polym Bull 17:231–237. https://doi.org/10.1007/BF00285355
Wada M, Okano T, Sugiyama J (1997) Synchrotron-radiated X-ray and neutron diffraction study of native. Cellulose 4:221–232. https://doi.org/10.1023/A:1018435806488
Ward K (1950) Crystallinity of cellulose and its significance for the fiber properties. Text Res J 20:363–372. https://doi.org/10.1177/004051755002000601
Warren BE (1990) X-Ray diffraction, Reprint edn. Dover Publications, New York
Watanabe K, Tabuchi M, Morinaga Y, Yoshinaga F (1998) Structural features and properties of bacterial cellulose produced in agitated culture. Cellulose 5:187–200. https://doi.org/10.1023/A:1009272904582
Young RA (ed) (1995) The Rietveld Method. In: OUP/International Union of Crystallography|International Union of Crystallography Monographs on Crystallography, pp. 5
Zhang Y, Inouye H, Crowley M et al (2016) Diffraction pattern simulation of cellulose fibrils using distributed and quantized pair distances. J Appl Crystallogr 49:2244–2248. https://doi.org/10.1107/S1600576716013297
Zhang S, Winter WT, Stipanovic AJ (2005) Water-activated cellulose-based electrorheological fluids. Cellulose 12:135–144. https://doi.org/10.1007/s10570-004-0345-2
Acknowledgments
The research work in this paper is supported by the Center for Lignocellulose Structure and Formation, an Energy Frontier Research Center funded by the U.S. Department of Energy, Office of Science, Office of Basic Energy Sciences under Award Number DE-SC0001090, and by the National Institute of Food and Agriculture, U.S. Department of Agriculture, Pennsylvania Agricultural Experiment Station 4602 under Accession Number 1009850. We thank two colleagues at Penn State University, Liza Wilson from Department of Biology for the training of ball milling and Nichole Wonderling from Materials Research Institute for the training and discussion on XRD analysis.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Yao, W., Weng, Y. & Catchmark, J.M. Improved cellulose X-ray diffraction analysis using Fourier series modeling. Cellulose 27, 5563–5579 (2020). https://doi.org/10.1007/s10570-020-03177-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10570-020-03177-8