Abstract
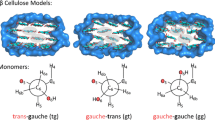
Molecular dynamics simulations of cellulose regularly sample a conformational space different to the crystal structures they were initiated from, with changes to the tilt of chains, expansion of the unit cell and variation in exocyclic group conformations. Given the differences in the structures sampled the question presents itself as to whether these simulations are sampling structures that resemble cellulose in planta. To investigate this question, we have performed MD simulations on different size and shaped Iα and Iβ cellulose microfibrils with the structures generated characterized with regards to changes in expansion, chain shift along the polymerization axis, tilt, exocyclic conformation and H-bonding. Structures were then input into a quantum mechanical NMR chemical shift calculation protocol with the resulting 13C chemical shifts compared to experimental data. Chemical shifts were shown to be strongly dependent on the exocyclic group conformation with the structures of Iα simulations more closely replicating experimental data than the Iβ simulations, especially at the C4 and C6 positions which suggests that the conformational space was not being accurately represented for the Iβ microfibrils. Despite this, peak sizes based on the sampling occupancy of exocyclic conformations from unrestrained simulations were found to replicate experimental peak sizes better than simulations where exocyclic groups of interior chains were restrained to the tg conformation, suggesting that exocyclic groups have greater freedom to sample different conformations than suggested by their crystal structures.




Similar content being viewed by others
Change history
14 May 2018
In the original publication of the article, the authors have not properly acknowledged the funding sources in the acknowledgments. Hence, the updated acknowledgment is provided below.
References
Adamo C, Barone V (1998) Exchange functionals with improved long-range behavior and adiabatic connection methods without adjustable parameters: the mPW and mPW1PW models. J Chem Phys 108:664–675. https://doi.org/10.1063/1.475428
Alekozai EM, GhattyVenkataKrishna PK, Uberbacher EC et al (2014) Simulation analysis of the cellulase Cel7A carbohydrate binding module on the surface of the cellulose Iβ. Cellulose 21:951–971. https://doi.org/10.1007/s10570-013-0026-0
Angles Dortoli T, Sjöberg NA, Vasiljeva P et al (2015) Temperature Dependence of Hydroxymethyl Group Rotamer Populations in Cellooligomers. J Phys Chem B 119:9559–9570. https://doi.org/10.1021/acs.jpcb.5b02866
Atalla RH, Van der Hart DL (1984) Native cellulose: a composite of two distinct crystalline forms. Science 80(23):283–285
Barnett CB, Naidoo KJ (2008) Stereoelectronic and solvation effects determine hydroxymethyl conformational preferences in monosaccharides. J Phys Chem B 112:15450–15459. https://doi.org/10.1021/jp8067409
Beckham GT, Matthews JF, Peters B et al (2011) Molecular-level origins of biomass recalcitrance: decrystallization free energies for four common cellulose polymorphs. J Phys Chem B 115:4118–4127. https://doi.org/10.1021/jp1106394
Bergenstråhle M, Thormann E, Nordgren N, Berglund LA (2009) Force pulling of single cellulose chains at the crystalline cellulose-liquid interface: a molecular dynamics study. Langmuir 25:4635–4642. https://doi.org/10.1021/la803915c
Brett CT (2000) Cellulose microfibrils in plants: biosynthesis, deposition, and integration into the cell wall. Int Rev Cytol 199:161–199. https://doi.org/10.1016/s0074-7696(00)99004-1
Bu L, Himmel ME, Crowley MF (2015) The molecular origins of twist in cellulose I-beta. Carbohydr Polym 125:146–152. https://doi.org/10.1016/j.carbpol.2015.02.023
Bučko T, Tunega D, Ángyán JG, Hafner J (2011) Ab initio study of structure and interconversion of native cellulose phases. J Phys Chem A 115:10097–10105. https://doi.org/10.1021/jp205827y
Bühl M, Kaupp M, Malkina OL, Malkin VG (1999) The DFT route to NMR chemical shifts. J Comput Chem 20:91–105. https://doi.org/10.1002/(sici)1096-987x(19990115)20:1<91::aid-jcc10>3.0.co;2-c
Busse-Wicher M, Gomes TCF, Tryfona T et al (2014) The pattern of xylan acetylation suggests xylan may interact with cellulose microfibrils as a two-fold helical screw in the secondary plant cell wall of Arabidopsis thaliana. Plant J 79:492–506. https://doi.org/10.1111/tpj.12575
Cheeseman JR, Trucks GW, Keith TA, Frisch MJ (1996) A comparison of models for calculating nuclear magnetic resonance shielding tensors. J Chem Phys 104:5497–5509. https://doi.org/10.1063/1.471789
Chen P, Nishiyama Y, Putaux J-L, Mazeau K (2014) Diversity of potential hydrogen bonds in cellulose I revealed by molecular dynamics simulation. Cellulose 21:897–908. https://doi.org/10.1007/s10570-013-0053-x
Ciesielski PN, Matthews JF, Tucker MP et al (2013) 3D electron tomography of pretreated biomass informs atomic modeling of cellulose microfibrils. ACS Nano 7:8011–8019. https://doi.org/10.1021/nn4031542
Conley K, Godbout L, Whitehead MA, Van De Ven TGM (2016) Origin of the twist of cellulosic materials. Carbohydr Polym 135:285–299. https://doi.org/10.1016/j.carbpol.2015.08.029
Devarajan A, Markutsya S, Lamm MH et al (2013) Ab initio study of molecular interactions in cellulose Iα. J Phys Chem B 117:10430–10443. https://doi.org/10.1021/jp406266u
Djahedi C, Berglund LA, Wohlert J (2015) Molecular deformation mechanisms in cellulose allomorphs and the role of hydrogen bonds. Carbohydr Polym 130:175–182. https://doi.org/10.1016/j.carbpol.2015.04.073
Elazzouzi-Hafraoui S, Nishiyama Y, Putaux JL et al (2008) The shape and size distribution of crystalline nanoparticles prepared by acid hydrolysis of native cellulose. Biomacromol 9:57–65. https://doi.org/10.1021/bm700769p
Fernandes AN, Thomas LH, Altaner CM et al (2011) Nanostructure of cellulose microfibrils in spruce wood. Proc Natl Acad Sci USA 108:E1195–E1203. https://doi.org/10.1073/pnas.1108942108
Funahashi R, Okita Y, Hondo H, et al (2017) Different conformations of surface cellulose molecules in native cellulose microfibrils revealed by layer-by-layer peeling. 0–3. https://doi.org/10.1021/acs.biomac.7b01173
Gomes TCF, Skaf MS (2012) Cellulose-builder: a toolkit for building crystalline structures of cellulose. J Comput Chem 33:1338–1346. https://doi.org/10.1002/jcc.22959
Gottlieb HE, Kotlyar V, Nudelman A (1997) NMR chemical shifts of common laboratory solvents as trace impurities. J Org Chem 62:7512–7515. https://doi.org/10.1021/jo971176v
Gross AS, Chu J-W (2010) On the molecular origins of biomass recalcitrance: the interaction network and solvation structures of cellulose microfibrils. J Phys Chem B 114:13333–13341. https://doi.org/10.1021/jp106452m
Gross AS, Bell AT, Chu J-W (2011) Thermodynamics of cellulose solvation in water and the ionic liquid 1-butyl-3-methylimidazolim chloride. J Phys Chem B 115:13433–13440. https://doi.org/10.1021/jp202415v
Guvench O, Greene SN, Kamath G et al (2008) Additive empirical force field for hexopyranose monosaccharides. J Comput Chem 29:2543–2564. https://doi.org/10.1002/jcc
Guvench O, Mallajosyula SS, Raman EP et al (2011) CHARMM additive all-atom force field for carbohydrate derivatives and its utility in polysaccharide and carbohydrate-protein modeling. J Chem Theory Comput 7:3162–3180. https://doi.org/10.1021/ct200328p
Hadden JA, French AD, Woods RJ (2013) Unraveling cellulose microfibrils: a twisted tale. Biopolymers 99:746–756. https://doi.org/10.1002/bip.22279
Hadden JA, French AD, Woods RJ (2014) Effect of microfibril twisting on theoretical powder diffraction patterns of cellulose I? Cellulose 21:879–884. https://doi.org/10.1007/s10570-013-0051-z
Hanley S, Revol J-F, Godbout L, Gray D (1997) Atomic force microscopy and transmission electron microscopy of cellulose from Micrasterias denticulata; evidence for a chiral helical microfibril twist. Cellulose 4:209–220. https://doi.org/10.1023/A:1018483722417
Hansen HS, Hünenberger PH (2011) A reoptimized GROMOS force field for hexopyranose-based carbohydrates accounting for the relative free energies of ring conformers, anomers, epimers, hydroxymethyl rotamers, and glycosidic linkage conformers. J Comput Chem 32:998–1032. https://doi.org/10.1002/jcc
Hanus J, Mazeau K (2006) The xyloglucan—cellulose assembly at the atomic scale. Biopolymers 82:59–73. https://doi.org/10.1002/bip
Heiner AP, Kuutti L, Teleman O (1998) Comparison of the interface between water and four surfaces of native crystalline cellulose by molecular dynamics simulations. Carbohydr Res 306:205–220. https://doi.org/10.1016/S0008-6215(97)10053-2
Hill JL, Hammudi MB, Tien M (2014) The arabidopsis cellulose synthase complex: a proposed hexamer of CESA trimers in an equimolar stoichiometry. Plant Cell Online 26:4834–4842. https://doi.org/10.1105/tpc.114.131193
Horii F, Hirai A, Kitamaru R (1983) Solid-state 13C-NMR study of conformations of oligosaccharides and cellulose conformation of CH2OH group about the exo-cyclic C–C bond. Polym Bull 10:357–361
Kafle K, Lee CM, Shin H et al (2015) Effects of delignification on crystalline cellulose in lignocellulose biomass characterized by vibrational sum frequency generation spectroscopy and X-ray diffraction. Bioenergy Res 8:1750–1758. https://doi.org/10.1007/s12155-015-9627-9
Kannam SK, Oehme DP, Doblin MS et al (2017) Hydrogen bonds and twist in cellulose microfibrils. Carbohydr Polym 175:433–439. https://doi.org/10.1016/j.carbpol.2017.07.083
Karadakov PB (2006) Ab initio calculation of NMR shielding constants. Modern magnetic resonance. Springer, Dordrecht, pp 63–70
Kim SH, Lee CM, Kafle K (2013) Characterization of crystalline cellulose in biomass: basic principles, applications, and limitations of XRD, NMR, IR, Raman, and SFG. Korean J Chem Eng 30:2127–2141. https://doi.org/10.1007/s11814-013-0162-0
Kirschner KN, Yongye AB, Tschampel SM et al (2007) GLYCAM06: a generalizable biomolecular force field. Carbohydrates. J Comput Chem 29:622–655. https://doi.org/10.1002/jcc
Kono H, Numata Y (2006) Structural investigation of cellulose Iα and Iβ by 2D RFDR NMR spectroscopy: determination of sequence of magnetically inequivalent d-glucose units along cellulose chain. Cellulose 13:317–326. https://doi.org/10.1007/s10570-005-9025-0
Kubicki JD, Mohamed MN-A, Watts HD (2013) Quantum mechanical modeling of the structures, energetics and spectral properties of Iα and Iβ cellulose. Cellulose 20:9–23. https://doi.org/10.1007/s10570-012-9838-6
Kubicki JD, Watts HD, Zhao Z, Zhong L (2014) Quantum mechanical calculations on cellulose–water interactions: structures, energetics, vibrational frequencies and NMR chemical shifts for surfaces of Iα and Iβ cellulose. Cellulose 21:909–926. https://doi.org/10.1007/s10570-013-0029-x
Kuttel M, Brady JW, Naidoo KJ (2002) Carbohydrate solution simulations: producing a force field with experimentally consistent primary alcohol rotational frequencies and populations. J Comput Chem 23:1236–1243. https://doi.org/10.1002/jcc.10119
Langan P, Petridis L, O’Neill HM et al (2014) Common processes drive the thermochemical pretreatment of lignocellulosic biomass. Green Chem 16:63. https://doi.org/10.1039/c3gc41962b
Larsson PT, Westlund P-O (2005) Line shapes in CP/MAS (13)C NMR spectra of cellulose I. Spectrochim Acta A Mol Biomol Spectrosc 62:539–546. https://doi.org/10.1016/j.saa.2005.01.021
Larsson PT, Wickholm K, Iversen T (1997) A CP/MAS 13C NMR investigation of molecular ordering in celluloses. Carbohydr Res 302:19–25
Lee CM, Kafle K, Park YB, Kim SH (2014) Probing crystal structure and mesoscale assembly of cellulose microfibrils in plant cell walls, tunicate tests, and bacterial films using vibrational Sum Frequency Generation (SFG) spectroscopy. Phys Chem Chem Phys 16:10844–10853. https://doi.org/10.1039/c4cp00515e
Lee CM, Kubicki JD, Fan B, et al (2015) Hydrogen-bonding network and OH stretch vibration of cellulose: comparison of computational modeling with polarized IR and SFG spectra. J Phys Chem B. https://doi.org/10.1021/acs.jpcb.5b08015
Li Y, Lin M, Davenport JW (2011) Ab initio studies of cellulose I: crystal structure, intermolecular forces, and interactions with water. J Phys Chem C 115:11533–11539. https://doi.org/10.1021/jp2006759
Lindner B, Petridis L, Schulz R, Smith JC (2013) Solvent-driven preferential association of lignin with regions of crystalline cellulose in molecular dynamics simulation. Biomacromol 14:3390–3398. https://doi.org/10.1021/bm400442n
Lodewyk MW, Siebert MR, Tantillo DJ (2012) Computational prediction of 1H and 13C chemical shifts: a useful tool for natural product, mechanistic, and synthetic organic chemistry. Chem Rev 112:1839–1862. https://doi.org/10.1021/cr200106v
Matthews JF, Bergenstråhle M, Beckham GT et al (2011a) High-temperature behavior of cellulose I. J Phys Chem B 115:2155–2166. https://doi.org/10.1021/jp1106839
Matthews JF, Himmel ME, Crowley MF (2011b) Conversion of cellulose Iα to Iβ via a high temperature intermediate (I-HT) and other cellulose phase transformations. Cellulose 19:297–306. https://doi.org/10.1007/s10570-011-9608-x
Matthews JF, Beckham GT, Bergenstråhle-Wohlert M et al (2012) Comparison of cellulose Iβ simulations with three carbohydrate force fields. J Chem Theory Comput 8:735–748. https://doi.org/10.1021/ct2007692
Maurer RJ, Sax AF, Ribitsch V (2013) Moleular simulation of surface reorganization and wetting in crystalline cellulose I and II. Cellulose 20:25–42. https://doi.org/10.1007/s10570-012-9835-9
Mazeau K, Vergelati C (2002) Atomistic modeling of the adsorption of benzophenone onto cellulosic surfaces. Langmuir 18:1919–1927. https://doi.org/10.1021/la010792q
Miyamoto H, Schnupf U, Brady JW (2014) Water structuring over the hydrophobic surface of cellulose. J Agric Food Chem 62:11017–11023. https://doi.org/10.1021/jf501763r
Moon RJ, Martini A, Nairn J et al (2011) Cellulose nanomaterials review: structure, properties and nanocomposites. Chem Soc Rev 40:3941. https://doi.org/10.1039/c0cs00108b
Nawrocki G, Cazade PA, Thompson D, Cieplak M (2015) Peptide recognition capabilities of cellulose in molecular dynamics simulations. J Phys Chem C 119:24404–24416. https://doi.org/10.1021/acs.jpcc.5b07118
Newman RH, Davies LM, Harris PJ (1996) Solid-State 13C nuclear magnetic resonance characterization of cellulose in the cell walls of arabidopsis thaliana leaves. Plant Physiol 111:475–485. https://doi.org/10.1104/pp.111.2.475
Newman RH, Hill SJ, Harris PJ (2013) Wide-angle x-ray scattering and solid-state nuclear magnetic resonance data combined to test models for cellulose microfibrils in mung bean cell walls. Plant Physiol 163:1558–1567. https://doi.org/10.1104/pp.113.228262
Nishiyama Y, Langan P, Chanzy H (2002) Crystal structure and hydrogen-bonding system in cellulose Iβ from synchrotron X-ray and neutron fiber diffraction. J Am Chem Soc 124:9074–9082. https://doi.org/10.1021/ja0257319
Nishiyama Y, Sugiyama J, Chanzy H, Langan P (2003) Crystal structure and hydrogen bonding system in cellulose Iα from synchrotron X-ray and neutron fiber diffraction. J Am Chem Soc 125:14300–14306. https://doi.org/10.1021/ja037055w
Nishiyama Y, Johnson GP, French AD (2012) Diffraction from nonperiodic models of cellulose crystals. Cellulose 19:319–336. https://doi.org/10.1007/s10570-012-9652-1
Nishiyama Y, Langan P, O’Neill H et al (2014) Structural coarsening of aspen wood by hydrothermal pretreatment monitored by small- and wide-angle scattering of X-rays and neutrons on oriented specimens. Cellulose 21:1015–1024. https://doi.org/10.1007/s10570-013-0069-2
Nixon BT, Mansouri K, Singh A et al (2016) Comparative structural and computational analysis supports eighteen cellulose synthases in the plant cellulose synthesis complex. Sci Rep 6:28696. https://doi.org/10.1038/srep28696
O’Neill H, Pingali SV, Petridis L et al (2017) Dynamics of water bound to crystalline cellulose. Sci Rep 7:11840. https://doi.org/10.1038/s41598-017-12035-w
Oehme DP, Doblin MS, Wagner J et al (2015a) Gaining insight into cell wall cellulose macrofibril organisation by simulating microfibril adsorption. Cellulose 22:3501–3520. https://doi.org/10.1007/s10570-015-0778-9
Oehme DP, Downton MT, Doblin MS et al (2015b) Unique aspects of the structure and dynamics of Iβ elementary cellulose microfibrils revealed by computational simulations. Plant Physiol 168:3–17. https://doi.org/10.1104/pp.114.254664
Paavilainen S, Rog T, Vattulainen I (2011) Analysis of twisting of cellulose nanofibrils in atomistic molecular dynamics simulations. J Phys Chem B 115:3747–3755. https://doi.org/10.1021/jp111459b
Park YB, Lee CM, Koo B-W et al (2013) Monitoring meso-scale ordering of cellulose in intact plant cell walls using sum frequency generation spectroscopy. Plant Physiol 163:907–913. https://doi.org/10.1104/pp.113.225235
Payne CM, Himmel ME, Crowley MF, Beckham GT (2011) Decrystallization of oligosaccharides from the cellulose Iβ surface with molecular simulation. J Phys Chem Lett 2:1546–1550. https://doi.org/10.1021/jz2005122
Pereira CS, Silveira RL, Dupree P, Skaf MS (2017) Effects of xylan side-chain substitutions on xylan-cellulose interactions and implications for thermal pretreatment of cellulosic biomass. Biomacromol 18:1311–1321. https://doi.org/10.1021/acs.biomac.7b00067
Peri S, Muthukumar L, Nazmul Karim M, Khare R (2012) Dynamics of cello-oligosaccharides on a cellulose crystal surface. Cellulose 19:1791–1806. https://doi.org/10.1007/s10570-012-9771-8
Petridis L, O’Neill HM, Johnsen M et al (2014) Hydration control of the mechanical and dynamical properties of cellulose. Biomacromol 15:4152–4159. https://doi.org/10.1021/bm5011849
Phillips JC, Braun R, Wang W et al (2005) Scalable molecular dynamics with NAMD. J Comput Chem 26:1781–1802. https://doi.org/10.1002/jcc.20289
Rassolov VA, Ratner MA, Pople JA et al (2001) 6-31G* basis set for third-row atoms. J Comput Chem 22:976–984. https://doi.org/10.1002/jcc.1058
Rockwell GD, Grindley TB (1998) Effect of solvation on the rotation of hydroxymethyl groups in carbohydrates. J Am Chem Soc 120:10953–10963. https://doi.org/10.1021/ja981958l
Sarotti AM, Pellegrinet SC (2009) A multi-standard approach for GIAO 13C NMR calculations. J Org Chem 74:7254–7260. https://doi.org/10.1021/jo901234h
Schreckenbach G, Ziegler T (1995) Calculation of NMR shielding tensors using gauge-including atomic orbitals and modern density functional theory. J Phys Chem 99:606–611. https://doi.org/10.1021/j100002a024
Shklyaev OE, Kubicki JD, Watts HD, Crespi VH (2014) Constraints on Iβ cellulose twist from DFT calculations of 13C NMR chemical shifts. Cellulose 21:3979–3991. https://doi.org/10.1007/s10570-014-0448-3
Silveira RL, Stoyanov SR, Kovalenko A, Skaf MS (2016) Cellulose aggregation under hydrothermal pretreatment conditions. Biomacromol. https://doi.org/10.1021/acs.biomac.6b00603
Srinivas G, Cheng X, Smith JC (2014) Coarse-grain model for natural cellulose fibrils in explicit water. J Phys Chem B 118:3026–3034. https://doi.org/10.1021/jp407953p
Stenutz R, Carmichael I, Widmalm G, Serianni AS (2002) Hydroxymethyl group conformation in saccharides: structural dependencies of 2 J HH, 3 J HH, and 1 J CH spin–spin coupling constants. J Org Chem 67:949–958. https://doi.org/10.1021/jo010985i
Sugiyama J, Vuong R, Chanzy H (1991) Electron diffraction study on the two crystalline phases occurring in native cellulose from an algal cell wall. Macromolecules 24:4168–4175. https://doi.org/10.1021/ma00014a033
Thibaudeau C, Stenutz R, Hertz B et al (2004) Correlated C–C and C–O bond conformations in saccharide hydroxymethyl groups: parametrization and application of redundant 1 H–1 H, 13 C–1 H, and 13 C–13 C NMR J-couplings. J Am Chem Soc 126:15668–15685. https://doi.org/10.1021/ja0306718
Thomas LH, Forsyth VT, Sturcová A et al (2013) Structure of cellulose microfibrils in primary cell walls from collenchyma. Plant Physiol 161:465–476. https://doi.org/10.1104/pp.112.206359
Thomas LH, Forsyth VT, Martel A et al (2015) Diffraction evidence for the structure of cellulose microfibrils in bamboo, a model for grass and cereal celluloses. BMC Plant Biol 15:153. https://doi.org/10.1186/s12870-015-0538-x
Vandavasi VG, Putnam DK, Zhang Q et al (2016) A structural study of CESA1 catalytic domain of arabidopsis cellulose synthesis complex: evidence for CESA trimers. Plant Physiol 170:123–135. https://doi.org/10.1104/pp.15.01356
Van der Hart DL, Atalla RH (1984) Studies of microstructure in native celluloses using solid-state 13C NMR. Macromolecules 17:1465–1472
Viëtor RJ, Newman RH, Ha M-A et al (2002) Conformational features of crystal-surface cellulose from higher plants. Plant J 30:721–731
Wang T, Hong M (2016) Solid-state NMR investigations of cellulose structure and interactions with matrix polysaccharides in plant primary cell walls. J Exp Bot 67:503–514. https://doi.org/10.1093/jxb/erv416
Wang T, Zabotina O, Hong M (2012) Pectin-cellulose interactions in the arabidopsis primary cell wall from two-dimensional magic-angle-spinning solid-state nuclear magnetic resonance. Biochemistry 51:9846–9856. https://doi.org/10.1021/bi3015532
Wang T, Yang H, Kubicki JD, Hong M (2016) Cellulose structural polymorphism in plant primary cell walls investigated by high-field 2D solid-state NMR spectroscopy and density functional theory calculations. Biomacromolecules. https://doi.org/10.1021/acs.biomac.6b00441
Watts HD, Mohamed MNA, Kubicki JD (2011) Comparison of multistandard and TMS-standard calculated NMR shifts for coniferyl alcohol and application of the multistandard method to lignin dimers. J Phys Chem B 115:1958–1970. https://doi.org/10.1021/jp110330q
Watts HD, Mohamed MNA, Kubicki JD (2014) A DFT study of vibrational frequencies and 13C NMR chemical shifts of model cellulosic fragments as a function of size. Cellulose 21:53–70. https://doi.org/10.1007/s10570-013-0128-8
Wiitala KW, Hoye TR, Cramer CJ (2006) Hybrid density functional methods empirically optimized for the computation of 13C and 1H chemical shifts in chloroform solution. J Chem Theory Comput 2:1085–1092. https://doi.org/10.1021/ct6001016
Wolinski K, Hinton JF, Pulay P (1990) Efficient implementation of the gauge-independent atomic orbital method for NMR chemical shift calculations. J Am Chem Soc 112:8251–8260. https://doi.org/10.1021/ja00179a005
Yamamoto H, Horii F, Odani H (1989) Structural changes of native cellulose crystals induced by annealing in aqueous alkaline and acidic solutions at high temperatures. Macromolecules 22:4130–4132. https://doi.org/10.1021/ma00200a058
Yang H, Wang T, Oehme D et al (2018) Structural factors affecting 13C NMR chemical shifts of cellulose: a computational study. Cellulose 25:23–36. https://doi.org/10.1007/s10570-017-1549-6
Zhang T, Zheng Y, Cosgrove DJ (2016) Spatial organization of cellulose microfibrils and matrix polysaccharides in primary plant cell walls as imaged by multichannel atomic force microscopy. Plant J 85:179–192. https://doi.org/10.1111/tpj.13102
Zhao G, Perilla JR, Yufenyuy EL et al (2013a) Mature HIV-1 capsid structure by cryo-electron microscopy and all-atom molecular dynamics. Nature 497:643–646. https://doi.org/10.1038/nature12162
Zhao Z, Shklyaev OE, Nili A et al (2013b) Cellulose microfibril twist, mechanics, and implication for cellulose biosynthesis. J Phys Chem A 117:2580–2589. https://doi.org/10.1021/jp3089929
Zhao Z, Crespi VH, Kubicki JD et al (2014) Molecular dynamics simulation study of xyloglucan adsorption on cellulose surfaces: effects of surface hydrophobicity and side-chain variation. Cellulose 21:1025–1039. https://doi.org/10.1007/s10570-013-0041-1
Acknowledgments
This work was partly funded by a grant from the Australia Research Council (ARC) to the ARC Centre of Excellence in Plant Cell Walls (DPO) [CE110001007]; the Victorian Life Sciences Computation Initiative (VLSCI) grant number “VR0319” on its Peak Computing Facility at the University of Melbourne, an initiative of the Victorian State Government; and the Center for Lignocellulose Structure and Formation, an Energy Frontier Research Center funded by the U.S. Department of Energy, Office of Science, Basic Energy Sciences under Award # DE- SC0001090. Parts of this work were completed while DPO was at IBM Research—Australia. Portions of this research were conducted with Advanced Cyber Infrastructure computational resources provided by the Institute for Cyber Science at The Pennsylvania State University (http://ics.psu.edu). We also thank Prof. Mei Hong for the experimental triple mutant intact Arabidopsis spectra.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Oehme, D.P., Yang, H. & Kubicki, J.D. An evaluation of the structures of cellulose generated by the CHARMM force field: comparisons to in planta cellulose. Cellulose 25, 3755–3777 (2018). https://doi.org/10.1007/s10570-018-1793-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10570-018-1793-4