Abstract
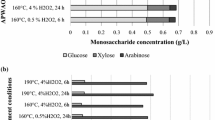
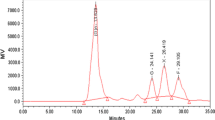
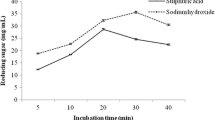
The complex and ordered arrangements of the lignocellulosic materials make them recalcitrant for their conversions to ethanol. Pretreatment is a crucial step in overcoming these hindrances. In this study, a 23-full factorial design of experiments optimization technique was applied on the alkaline peroxide oxidation pretreatments of rice husks biomass. The low–high levels of the influencing variables on pretreatments were; temperature (100–120 °C), time (1–2 h), % (v/v)H2O2 concentration (1–3%). Under the prevailing pretreatments, the optimum conditions were predicted and validated to be 109 °C, 2 h, and 1.38% H2O2 which yielded 56% (w/w) cellulose content, 55% (w/w) hemicellulose solubilization, and 48% (w/w) lignin removal. At the established optimum pretreatment conditions, and considering variations in biomass and enzymes loadings, maximum reducing sugars production was 205 mg/g dry biomass at different enzymatic hydrolysis conditions of 3% biomass loading, hydrolysis temperature of 45 °C, hydrolysis time of 24 h, and 35 FPU/g cellulose enzyme loading. The highest cellulose conversion of 33% yielded 24 g/L ethanol at the end of the first day of saccharification and fermentation. Physical, structural, and morphological investigations on raw and treated materials using tools such as stereomicroscopy, scanning electron microscopy, and fourier transform infrared spectroscopy further revealed the effectiveness of chosen method on rice husks biomass.








Similar content being viewed by others
References
Agnemo R, Gellerstedt G (1979) Reactions of lignin with alkaline hydrogen peroxide. Part II. Factors influencing the decomposition of phenolic structures. Acta Chem Scand B33:337–342
Akerholm M, Hinterstoisser B, Salmen L (2004) Characterization of the crystalline structure of cellulose using static and dynamic FT-IR spectroscopy. Carbohydr Res 339:569–578
Andrić P, Meyer AS, Jensen PA, Dam-Johansen K (2010) Reactor design for minimizing product inhibition during enzymatic lignocellulosic hydrolysis II. Quantification of inhibition and suitability of membrane reactors. Biotechnol Adv 28:407–425
Asadieraghi M, Ashri WM, Daud W (2014) Characterization of lignocellulosic biomass thermal degradation and physiochemical structure: effect of demineralization by diverse acid solutions. Energy Convers Manag 82:71–82
ASTM D2015. Standard test method for gross caloric value of coal and coke by adiabatic bomb calorimeter. https://www.astm.org/Standards/D2015.htm
Awosusi AA, Ayeni A, Adeleke R, Daramola MO (2017a) Effect of water of crystallization on the dissolution efficiency of molten zinc chloride hydrate salts during the pretraetment of corncob biomass. J Chem Tech Biotechnol 92:2468–2476
Awosusi AA, Ayeni A, Adeleke R, Daramola MO (2017b) Biocompositional and thermodecompositional analysis of South African agro-waste corncob and husk towards production of biocommodities. Asia Pac J Chem Eng 12:960–968
Ayeni AO, Daramola MO (2017) Lignocellulosic biomass waste beneficiation: evaluation of oxidative and non-oxidative pretreatment methodologies of South African corn cob. J Environ Chem Eng 5:1771–1779
Ayeni AO, Banerjee S, Omoleye JA, Hymore FK, Giri BS, Deshmukh SC, Pandey RA, Mudliar SN (2013a) Optimization of pretreatment conditions using full factorial design and enzymatic convertibility of shea tree sawdust. Biomass Bioenergy 48:130–138
Ayeni AO, Hymore FK, Mudliar SN, Deskmukh SC, Satpute DB, Omoleye JA, Pandey RA (2013b) Hydrogen peroxide and lime based oxidative pretreatment of wood waste to enhance enzymatic hydrolysis for a biorefinery: process parameters optimization using response surface methodology. Fuel 106:187–194
Ayeni AO, Omoleye JA, Mudliar SN, Hymore FK, Pandey RA (2014) Utilization of lignocellulosic waste for ethanol production: enzymatic digestibility and fermentation of pretreated shea tree sawdust. Korean J Chem Eng 31:1180–1186
Ayeni AO, Omoleye JA, Hymore FK, Pandey RA (2016a) Effective alkaline peroxide oxidation pretreatment of shea tree sawdust for the production of biofuels: kinetics of delignification and enzymatic conversion to sugar and subsequent production of ethanol by fermentation using Saccharomyces cerevisiae. Braz J Chem Eng 33:33–45
Ayeni AO, Ogu R, Awosusi AA, Daramola MO (2016b) Alkaline peroxide oxidation pretreatment of corn cob and rice husks for bioconversion into bio-commodities: Part A-Enzymatic converstibility of pretreated rice husks to reducing sugar. In: 24th European biomass conference and exhibition, pp 1225–1232. Amsterdam, Netherlands
Bailey CW, Dence CW (1969) Reactions of alkaline hydrogen peroxide with softwood lignin model compounds. Tappi J 52:491–500
Banerjee S, Sen R, Pandey R, Chakrabarti T, Satpute D, Giri B, Mudliar SN (2009) Evaluation of wet air oxidation as a pretreatment strategy for bioethanol production from rice husk and process optimization. Biomass Bioenergy 33:1680–1686
Bartos C, Kukovecz Á, Ambrus R, Farkas G, Radacsi N, Szabó-Révész P (2015) Comparison of static and dynamic sonication as process intensification for particle size reduction using a factorial design. Chem Eng Process 87:26–34
Basu P (2010) Biomass gasification and pyrolysis: practical design and theory. Elsevier, Oxford
Bennet C (1971) Spectrophotometric acid dichromate method for the determination of ethyl alcohol. Am J Med Technol 37:217–220
Bian J, Peng F, Peng X-P, Peng P, Xu F, Sun R-C (2012) Acetic acid enhanced purification of crude cellulose from sugarcane bagasse: structural and morphological characterization. Bioresources 7:4626–4639
Binder JB, Raines RT (2010) Fermentable sugars by chemical hydrolysis of biomass. Proc Natl Acad Sci USA 107:4516–4521
Blasi CD, Signorelli G, Russo CD, Rea G (1999) Product distribution from pyrolysis of wood and agricultural residues. Ind Eng Chem Res 38:2216–2224
Boie W (1957) Vom Brennstoff zum Rauchgas, in: Feuerungstechnisches Rechnen mit Brennstoffkenngr¨oßen und seine Vereinfachung mitMitteln der Statistik, Teubner, Leipzig
Buzala K, Przybysz P, Rosicka-Kaczmarek J, Kalinowska H (2015) Production of glucose-rich enzymatic hydrolysates from cellulosic pulps. Cellulose 22:663–674
Chang VS, Nagwani M, Holtzapple MT (1998) Lime pretreatment of crop residues bagasse and wheat straw. Appl Biochem Biotechnol 74:135–159
Chundawat SPS, Donohoe BS, Sousa LD, Elder T, Agarwal UP, Lu F, Ralph J, Himmel ME, Balan V, Dale BE (2011) Multi-scale visualization and characterization of lignocellulosic plant cell wall deconstruction during thermochemical pretreatment. Energy Environ Sci 4:973–984
Ciesielski PN, Wang W, Chen X, Vinzant TB, Tucker MP, Decker SR, Himmel ME, Johnson DK, Donohoe BS (2014) Effect of mechanical disruption on the effectiveness of three reactors used for dilute acid pretreatment of corn stover Part 2: morphological and structural substrate analysis. Biotechnol Biofuels 7:47
Dagnino EP, Chamorro ER, Romano SD, Felissia FE, Area MC (2013) Optimization of the acid pretreatment of rice hulls to obtain fermentable sugars for bioethanol production. Ind Crops Prod 42:363–368
Demirbas A (1997) Calculation of high heating values of biomass fuels. Fuel 76:431–434
Donohoe BS, Decker SR, Tucker MP, Himmel ME, Vinzant TB (2008) Visualizing lignin coalescence and migration through maize cell walls following thermochemical pretreatment. Biotechnol Bioeng 101:913–925
Dowe N, McMillan J (2008) SSF experimental protocols: Lignocellulosic biomass hydrolysis and fermentation LAP. NREL/TP-510-42630. Contract No.: DE-AC36-99-G010337
El-Sayed SA, Mostafa ME (2015) Kinetic parameters determination of biomass pyrolysis fuels using TGA and DTA techniques. Waste Biomass Valoris 6:401–415
Fannie PE, Okafor J, Roberson C (1998) Determination of insoluble solids of pretreated biomass material. LAP-18. Version 09-23-1998
Forney LJ, Reddy CA, Tien M, Aust SD (1982) The involvement of hydroxyl radical derived from hydrogen peroxide in lignin degradation by the white rot fungus phanerochaete chrysosporium. J Biol Chem 257:11455–11462
García R, Pizarro C, Lavín AG, Bueno JL (2014) Spanish biofuels heating value estimation. Part 1: Ultimate analysis data. Fuel 117:1130–1138
Gould JM (1985) Studies on the mechanism of alkaline peroxide delignification of agricultural residues. Biotechnol Bioeng 27:225–231
Hsu T, Guo G, Chen W, Hwang W (2010) Effect of dilute acid pretreatment of rice straw on structural properties and enzymatic hydrolysis. Bioresour Technol 101:4907–4913
Jenkins BM, Ebeling JM (1985) Correlations of physical and chemical properties of terrestrial biomass with conversion. In: Proceedings of 1985 symposium energy from biomass and waste IX IGT, p 271, California
Jeya M, Zhang Y, Kim I, Lee J (2009) Enhanced saccharification of alkali-treated rice straw by cellulase from Trametes hirsuta and statistical optimization of hydrolysis conditions by RSM. Bioresour Technol 100:5155–5161
Jung KW, Kim DH, Kim HW, Shin HS (2011) Optimization of combined (acid + thermal) pretreatment for fermentative hydrogen production from Laminaria japonica using response surface methodology (RSM). Int J Hydrog Energy 36:9626–9631
Kaiser S, Verza SG, Moraes RC, Pittol V, Peñaloza EMC, Pavei C, Ortega GG (2013) Extraction optimization of polyphenols, oxindole alkaloids and quinovic acid glycosides from cat’s claw bark by Box-Behnken design. Ind Crops Prod 48:153–161
Kim S, Dale BE (2004) Global potential bioethanol production from wasted crops and crop residues. Biomass Bioenergy 26:361–375
Kutsuki H, Gold MH (1982) Generation of hydroxyl radical and its involvement in lignin degradation by Phanerochaete chrysosporium. Biochem Biophys Res Cornrnun 109:320–327
Lachenal D, de Choudens C, Monzie P (1980) Hydrogen peroxide as a delignifying agent. Tappi J 63:119–122
Lee JH, Lim SL, Song YS, Kang SW, Park C, Kim SW (2007) Optimization of culture medium for lactosucrose (4G-β-D-Galactosylsucrosw) production by Sterigmatomyces elviae mutant using statistical analysis. J Microbiol Biotechnol 17:1996–2004
Li S, Xu S, Liu S, Yang C, Lu Q (2004) Fast pyrolysis of biomass in free—fall reactor for hydrogen—rich gas. Fuel Process Technol 85:1201–1211
Mani T, Murugan P, Abedi J, Mahinpey N (2010) Pyrolysis of wheat straw in a thermogravimetric analyser: effect of particle size and heating rate on devolatilization and estimation of global kinetics. Chem Eng Res Des 88:952–958
McGinnis GD, Prince SE, Biermann CJ, Lowrimore JF (1984) Wet oxidation of model carbohydrate compounds. Carbohyd Res 128:51–60
Miller GL (1959) Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal Chem 31:426–428
Nelson ML, O’Connor RT (1964) Relation of certain infrared bands to cellulose crystallinity and crystal lattice type. Part II. A new infrared ratio for estimation of crystallinity in cellulose I and II. J Appl Polym Sci 8:1325–1341
Nikzad M, Movagharnejad K, Najafpour GD, Talebnia F (2013) Comparative studies of effect of pretreatment of rice husk for enzymatic digestibility and bioethanol production. Int J Eng 26:455–464
O’Sullivan AC (1997) Cellulose: the structure slowly unravels. Cellulose 4:173–207
Onoji SE, Iyuke SE, Igbafe AI, Daramola MO (2017) Hevea brasiliensis (rubber seed) oil: modelling and optimization of extraction process parameters using response surface methodology and artificial neural network techniques. Biofuels. https://doi.org/10.1080/17597269.2017.1338122
Park S, Baker JO, Himmel ME, Parilla PA, Johnson DK (2010) Cellulose crystallinity index: measurement techniques and their impact on interpreting cellulase performance. Biotechnol Biofuels 3:10
Permchart W, Kouprianov VI (2004) Emission performance and combustion efficiency of a conical fluidized-bed combustor firing various biomass fuels. Bioresour Technol 92:83–91. https://doi.org/10.1016/j.biortech.2003.07.005
Saha BC, Cotta MA (2007) Enzymatic saccharification and fermentation of alkaline peroxide pretreated rice hulls to ethanol. Enzyme Microb Technol 41:528–532
Sarita CR, Filho RM, Costa AC (2009) Lime pretreatment of sugarcane bagasse for bioethanol production. Appl Biochem Biotechnol 153:139–150
Sluiter A, Hames B, Ruiz R, Scarlata C, Sluiter J, Templeton D (2008) Determination of Ash in Biomass. LAP. NREL/TP 510-42622
Sluiter A, Hames B, Ruiz R, Scarlata C, Sluiter J, Templeton D, Crocker D (2012) Determination of structural carbohydrates and lignin in biomass. LAP. NREL/TP 510-42618. Version 08-03-2012
Spiridon I, Teacã C, Bodîrlãu R (2011) Structural changes evidenced by FTIR spectroscopy in cellulosic materials after pretreatment with ionic liquid and enzymatic hydrolysis. Bioresources 6:400–413
Stasolla C, Scott J, Egertsdotter U, Kadla J, O’Malley D, Sederoff R, Zyl L (2003) Analysis of lignin produced by cinnamyl alcohol dehydrogenase-deficient Pinus taeda cultured cells. Plant Physiol Biochem 41:439–445
Tengborg C, Galbe M, Zacchi G (2001) Influence of enzyme loading and physical parameters on the enzymatic hydrolysis of steam pretreated softwood. Biotechnol Progress 17:110–117
Tokoh C, Takabe K, Fujita M, Saiki H (1998) Cellulose synthesized by Azetobacter xylinum in the presence of acetyl glucomannan. Cellulose 5:249–261
Varanasi P, Singh P, Auer M, Adams PD, Simmons BA, Singh S (2013) Survey of renewable chemicals produced from lignocellulosic biomass during ionic liquid pretreatment. Biotechnol Biofuels 6:14
Viamajala S, McMillan JD, Schell DJ, Elander RT (2009) Rheology of corn stover slurries at high solids concentrations–Effects of saccharification and particle size. Bioresour Technol 100:925–934
Wang K, Yang H, Wang W, Sun R (2013) Structural evaluation and bioethanol production by simultaneous saccharification and fermentation with biodegraded triploid poplar. Biotechnol Biofuels 6:42
Wright MM, Daugaard DE, Satrio JA, Brown RC (2010) Techno-economic analysis of biomass fast pyrolysis to transportation fuels. Fuel 89:S2–S10
Xing Y, Bu L, Sun D, Liu Z, Liu S, Jiang J (2016) Enhancement of high-solid enzymatic hydrolysis and fermentation of furfural residues by addition of Gleditsia saponin. Fuel 177:142–147
Yang B, Wyman CE (2008) Pretreatment: the key to unlocking low-cost cellulosic ethanol. Biofuels Bioprod Biorefin 2:26–40
Yin C (2011) Prediction of higher heating values of biomass from proximate and ultimate analyses. Fuel 90:1128–1132
Zhang Q, Cai W (2008) Enzymatic hydrolysis of alkali-pretreated rice straw by Trichoderma reesei ZM4-F3. Biomass Bioenergy 32:1130–1135
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ayeni, A.O., Daramola, M.O., Sekoai, P.T. et al. Statistical modelling and optimization of alkaline peroxide oxidation pretreatment process on rice husk cellulosic biomass to enhance enzymatic convertibility and fermentation to ethanol. Cellulose 25, 2487–2504 (2018). https://doi.org/10.1007/s10570-018-1714-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10570-018-1714-6