Abstract
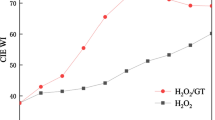
O-phthalic anhydride (PA) was developed as a low-temperature activator in the H2O2 bleaching system for cotton fabric. The performance of the H2O2/PA bleaching system was investigated by measuring the CIE whiteness index (WI) of the bleached cotton fabric, H2O2 decomposition rate and bursting strength, respectively. The effects of experimental conditions, including PA dosage, bleaching time, bleaching temperature and NaOH concentration, were discussed in the H2O2/PA bleaching system. Upon addition of PA, the WI and H2O2 decomposition rate increased significantly at 70 °C. The results showed that the bleaching system had a promising application prospect. Compared with the H2O2 system, the H2O2/PA system significantly promoted 1O2 generation under the same alkali condition by using 9,10-dimethylanthracene as a fluorescent probe for 1O2. And the generation of large amount of 1O2 did not obviously affect the bleaching performance in the H2O2/PA system. By using benzenepentacarboxylic acid as a fluorescent probe for HO· detection, it was found that the fluorescence intensity was enhanced 14 folds in the presence of PA, indicating that PA could strongly promote HO· generation. By using dimethyl sulfoxide as a scavenger to control HO· concentration, it was found that the WI of the fabric was closely related to the HO· concentration, revealing that the HO· could play an important role in the bleaching process. Phthalic acid was also added to the H2O2 bleaching system to investigate potential bleaching route of the H2O2/PA system. Based on these experimental results, a possible bleaching mechanism of the H2O2/PA system was proposed.











Similar content being viewed by others
References
Bianchetti GO, Devlin CL, Seddon KR (2015) Bleaching systems in domestic laundry detergents: a review. RSC Adv 5:65365–65384. https://doi.org/10.1039/c5ra05328e
Botsivali M, Evans DF (1979) A new trap for singlet oxygen in aqueous solution. J Chem Soc, Chem Commun. https://doi.org/10.1039/C39790001114
Cai JY, Evans DJ, Smith SM (2001) Bleaching of natural fibers with TAED and NOBS activated peroxide systems. AATCC Rev 1:31–34
Chen W, Wang L, Wang D, Zhang J, Sun C, Xu C (2016) Recognizing a limitation of the TBLC-activated peroxide system on low-temperature cotton bleaching. Carbohydr Polym 140:1–5. https://doi.org/10.1016/j.carbpol.2015.12.013
Dannacher JJ (2006) Catalytic bleach: most valuable applications for smart oxidation chemistry. J Mol Catal A: Chem 251:159–176. https://doi.org/10.1016/j.molcata.2006.02.031
Dannacher J, Schlenker W (1996) The mechanism of hydrogen peroxide bleaching. Text Chem Color 28:24–28
Ek M, Gierer J, Jansbo K, Reitberger T (1989) Study on the selectivity of bleaching with oxygen-containing species. Holzforschung 43:391–396. https://doi.org/10.1515/hfsg.1989.43.6.391
Eren H, Avinc O, Erişmiş B, Eren S (2014) Ultrasound-assisted ozone bleaching of cotton. Cellulose 21:4643–4658. https://doi.org/10.1007/s10570-014-0420-2
Fei X, Yao J, Du J, Sun C, Xiang Z, Xu C (2015) Analysis of factors affecting the performance of activated peroxide systems on bleaching of cotton fabric. Cellulose 22:1379–1388. https://doi.org/10.1007/s10570-015-0550-1
Gomes A, Fernandes E, Lima JLFC (2005) Fluorescence probes used for detection of reactive oxygen species. J Biochem Biophys Methods 65:45–80. https://doi.org/10.1016/j.jbbm.2005.10.003
Hickman WS (2002) Peracetic acid and its use in fibre bleaching. Rev Prog Color Relat Top 32(1):13–27. https://doi.org/10.1111/j.1478-4408.2002.tb00247.x
Hofmann J, Just G, Pritzkow W, Schmidt H (1992) Bleaching activators and the mechanism of bleaching activation. J Prakt Chem Chem Ztg 334:293–297. https://doi.org/10.1002/prac.19923340402
Hong KH, Sun G (2011) Photoactive antibacterial cotton fabrics treated by 3,3′,4,4′-benzophenonetetracarboxylic dianhydride. Carbohydr Polym 84:1027–1032. https://doi.org/10.1016/j.carbpol.2010.12.062
Hou A, Sun G (2013) Multifunctional finishing of cotton fabrics with 3,3′,4,4′-benzophenone tetracarboxylic dianhydride: reaction mechanism. Carbohydr Polym 95:768–772. https://doi.org/10.1016/j.carbpol.2013.02.027
Hou A, Feng G, Zhuo J, Sun G (2015) UV light-induced generation of reactive oxygen species and antimicrobial properties of cellulose fabric modified by 3,3′,4,4′-benzophenone tetracarboxylic acid. ACS Appl Mater Interfaces 7:27918–27924. https://doi.org/10.1021/acsami.5b09993
Iamazaki E, Orblin E, Fardim P (2013) Topochemical activation of pulp fibres. Cellulose 20:2615–2624. https://doi.org/10.1007/s10570-013-0016-2
Jegannathan KR, Nielsen PH (2013) Environmental assessment of enzyme use in industrial production—a literature review. J Clean Prod 42:228–240. https://doi.org/10.1016/j.jclepro.2012.11.005
Kim S, Tachikawa T, Fujitsuka M, Majima T (2014) Far-red fluorescence probe for monitoring singlet oxygen during photodynamic therapy. J Am Chem Soc 136:11707–11715. https://doi.org/10.1021/ja504279r
Lee J, Hinks D, Lim S-H, Hauser P (2010) Hydrolytic stability of a series of lactam-based cationic bleach activators and their impact on cellulose peroxide bleaching. Cellulose 17:671–678. https://doi.org/10.1007/s10570-009-9390-1
Liu K, Zhang X, Yan K (2017) Low-temperature bleaching of cotton knitting fabric with H2O2/PAG system. Cellulose 24:1555–1561. https://doi.org/10.1007/s10570-016-1167-8
Long X, Xu C, Du J, Fu S (2013) The TAED/H2O2/NaHCO3 system as an approach to low-temperature and near-neutral pH bleaching of cotton. Carbohydr Polym 95:107–113. https://doi.org/10.1016/j.carbpol.2013.02.061
Luo X, Sui X, Yao J, Fei X, Du J, Sun C, Xiang Z, Xu C, Wang S (2015) Performance modelling of the TBCC-activated peroxide system for low-temperature bleaching of cotton using response surface methodology. Cellulose 22:3491–3499. https://doi.org/10.1007/s10570-015-0741-9
Milne NJ (1998) Oxygen bleaching systems in domestic laundry. J Surfactants Deterg 1:253–261. https://doi.org/10.1007/s11743-998-0029-z
Qi H, Pan J, Qing FL, Yan K, Sun G (2016) Anti-wrinkle and UV protective performance of cotton fabrics finished with 5-(carbonyloxy succinic)-benzene-1,2,4-tricarboxylic acid. Carbohydr Polym 154:313–319. https://doi.org/10.1016/j.carbpol.2016.05.108
Reinhardt G, Borchers G (2008) Application of bleaching detergent formulations. In: Zoller U (ed) Handbook of detergents, part E: applications. CRC Press, Boca Raton, pp 375–418
Si F, Yan K, Zhang X (2014a) Study on H2O2/TAED and H2O2/TBCC bleaching mechanism related to hydroxyl radical with a fluorescent probe. Carbohydr Polym 103:581–586. https://doi.org/10.1016/j.carbpol.2013.12.052
Si F, Zhang X, Yan K (2014b) The quantitative detection of HO˙ generated in a high temperature H2O2 bleaching system with a novel fluorescent probe benzenepentacarboxylic acid. RSC Adv 4(12):5860–5866. https://doi.org/10.1039/C3RA45975F
Tang P, Sun G (2017) Generation of hydroxyl radicals and effective whitening of cotton fabrics by H2O2 under UVB irradiation. Carbohydr Polym 160:153–162. https://doi.org/10.1016/j.carbpol.2016.12.062
Wang S, Li S, Zhu Q, Yang CQ (2014) A novel low temperature approach for simultaneous scouring and bleaching of knitted cotton fabric at 60 °C. Ind Eng Chem Res 53:9985–9991. https://doi.org/10.1021/ie500062f
Wang G, Umbuzeiro GdA, Vendemiatti JA, de Oliveira AC, Vacchi FI, Hussain M, Hauser PJ, Freeman HS, Hinks D (2017) Synthesis, characterization, and toxicological properties of new cationic bleach activators. J Surfactants Deterg 20:277–285. https://doi.org/10.1007/s11743-016-1899-3
Wasserman HH, Scheffer JR, Cooper JL (1972) Singlet oxygen reactions with 9,10-diphenylanthracene peroxide. J Am Chem Soc 94:4991–4996
Xu C, Long X, Du J, Fu S (2013) A critical reinvestigation of the TAED-activated peroxide system for low-temperature bleaching of cotton. Carbohydr Polym 92:249–253. https://doi.org/10.1016/j.carbpol.2012.08.088
Acknowledgments
This work was supported by the Fundamental Research Funds for the Central Universities of Donghua University (Grant No. CUSF-DH-D-2017052). The authors gratefully acknowledge Dr. Chunyan Hu for the experimental equipment and Dr. Bolin Ji for statistical analyses. The first author thanks the scholarship support from China Scholarship Council (CSC).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Liu, K., Zhang, X. & Yan, K. Development of o-phthalic anhydride as a low-temperature activator in H2O2 bleaching system for cotton fabric. Cellulose 25, 859–867 (2018). https://doi.org/10.1007/s10570-017-1578-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10570-017-1578-1