Abstract
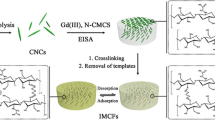
We successfully prepared three-dimensionally interconnected macroporous imprinted chitosan films (3DIM-IFs) by template-assisted assembly. Imprinted chitosan films exhibiting an interconnected macroporous structure are used as adsorbents for efficient and selective adsorption of gadolinium ions (Gd(III)). Saturation adsorption capacity of 3DIM-IFs for Gd(III) is up to 51.36 mg g−1 at 298 K, which is significantly higher than adsorption capacities for most reported Gd(III) imprinted adsorbents during recent years. Because of highly selective imprinted sites, imprinted films possess significant selectivity of Gd(III) than other rare earth ions. Moreover, 3DIM-IFs can be easily and rapidly retrieved without the need of additional centrifugation or filtration, greatly facilitating the separation process. Reusability tests demonstrated that the materials can be repeatedly used without significant loss in adsorption capacity, enhancing their potential application for recovery of Gd(III).








Similar content being viewed by others
References
Addo Ntim S, Mitra S (2011) Removal of trace arsenic to meet drinking water standards using iron oxide coated multiwall carbon nanotubes. J Chem Eng Data 56:2077–2083
Alizadeh T, Amjadi S (2013) Synthesis of nano-sized Eu 3+-imprinted polymer and its application for indirect voltammetric determination of europium. Talanta 106:431–439
Alonso E, Sherman AM, Wallington TJ, Everson MP, Field FR, Roth R, Kirchain RE (2012) Evaluating rare earth element availability: a case with revolutionary demand from clean technologies. Environ Sci Technol 46:3406–3414
Binnemans K, Jones PT, Blanpain B, Van Gerven T, Yang Y, Walton A, Buchert M (2013) Recycling of rare earths: a critical review. J Clean Prod 51:1–22
Branger C, Meouche W, Margaillan A (2013) Recent advances on ion-imprinted polymers. React Funct Polym 73:859–875
Fan J, Wang T, Yu C, Tu B, Jiang Z, Zhao D (2004) Ordered, nanostructured tin-based oxides/carbon composite as the negative-electrode material for lithium-ion batteries. Adv Mater 16:1432–1436
Fu J, Chen L, Li J, Zhang Z (2015) Current status and challenges of ion imprinting. J Mater Chem A 3:13598–13627
Gao B, Zhang Y, Xu Y (2014) Study on recognition and separation of rare earth ions at picometre scale by using efficient ion-surface imprinted polymer materials. Hydrometallurgy 150:83–91
He J, Lu Y, Luo G (2014) Ca (II) imprinted chitosan microspheres: an effective and green adsorbent for the removal of Cu (II), Cd (II) and Pb(II) from aqueous solutions. Chem Eng J 244:202–208
Kulaksız S, Bau M (2013) Anthropogenic dissolved and colloid/nanoparticle-bound samarium, lanthanum and gadolinium in the Rhine River and the impending destruction of the natural rare earth element distribution in rivers. Earth Planet Sci Lett 362:43–50
Kyzas GZ, Siafaka PI, Pavlidou EG, Chrissafis KJ, Bikiaris DN (2015) Synthesis and adsorption application of succinyl-grafted chitosan for the simultaneous removal of zinc and cationic dye from binary hazardous mixtures. Chem Eng J 259:438–448
Li K et al (2015) Selective adsorption of Gd3+ on a magnetically retrievable imprinted chitosan/carbon nanotube composite with high capacity. ACS Appl Mater Interfaces 7:21047–21055
Moss R et al (2013) Critical metals in the path towards the decarbonisation of the EU energy sector assessing rare metals as supply-chain bottlenecks in low-carbon energy technologies JRC report EUR 25994
Mou F, Guan J, Ma H, Xu L, Shi W (2012) Magnetic iron oxide chestnutlike hierarchical nanostructures: preparation and their excellent arsenic removal capabilities. ACS Appl Mater Interfaces 4:3987–3993
Santos D, Neto C, Fonseca J, Pereira M (2008) Chitosan macroporous asymmetric membranes—preparation, characterization and transport of drugs. J Membr Sci 325:362–370
Stöber W, Fink A, Bohn E (1968) Controlled growth of monodisperse silica spheres in the micron size range. J Colloid Interface Sci 26:62–69. doi:10.1016/0021-9797(68)90272-5
Sun Y, Shao D, Chen C, Yang S, Wang X (2013) Highly efficient enrichment of radionuclides on graphene oxide-supported polyaniline. Environ Sci Technol 47:9904–9910
Uda T, Jacob KT, Hirasawa M (2000) Technique for enhanced rare earth separation. Science (Washington, DC) 289:2326–2329. doi:10.1126/science.289.5488.2326
Vigneau O, Pinel C, Lemaire M (2001) Ionic imprinted resins based on EDTA and DTPA derivatives for lanthanides (III) separation. Anal Chim Acta 435:75–82
Wang S, Zhai Y-Y, Gao Q, Luo W-J, Xia H, Zhou C-G (2013) Highly efficient removal of acid red 18 from aqueous solution by magnetically retrievable chitosan/carbon nanotube: batch study, isotherms, kinetics, and thermodynamics. J Chem Eng Data 59:39–51
Yang F, Liu H, Qu J, Paul Chen J (2011) Preparation and characterization of chitosan encapsulated Sargassum sp. biosorbent for nickel ions sorption. Bioresour Technol 102:2821–2828. doi:10.1016/j.biortech.2010.10.038
Yang S, Han C, Wang X, Nagatsu M (2014) Characteristics of cesium ion sorption from aqueous solution on bentonite- and carbon nanotube-based composites. J Hazard Mater 274:46–52
Yao Y et al (2011) Interconnected silicon hollow nanospheres for lithium-ion battery anodes with long cycle life. Nano Lett 11:2949–2954
Yu X-Y et al (2011a) Adsorption of lead (II) on O2-plasma-oxidized multiwalled carbon nanotubes: thermodynamics, kinetics, and desorption. ACS Appl Mater Interfaces 3:2585–2593
Yu Y, Gu L, Lang X, Zhu C, Fujita T, Chen M, Maier J (2011b) Li storage in 3D nanoporous Au-supported nanocrystalline tin. Adv Mater 23:2443–2447
Zhang J et al (2009) Creation of three-dimensionally ordered macroporous Au/CeO2 catalysts with controlled pore sizes and their enhanced catalytic performance for formaldehyde oxidation. Appl Catal B 91:11–20. doi:10.1016/j.apcatb.2009.05.001
Zheng X, Wang C, Dai J, Shi W, Yan Y (2015) Design of mesoporous silica hybrid materials as sorbents for the selective recovery of rare earth metals. J Mater Chem A 3:10327–10335. doi:10.1039/c4ta06860b
Zheng X, Liu E, Zhang F, Yan Y, Pan J (2016) Efficient adsorption and separation of dysprosium from NdFeB magnets in acidic system by ion imprinted mesoporous silica sealed in dialysis bag. Green Chem
Acknowledgments
This work was financially supported by the National Natural Science Foundation of China (No. 21576120, No. 21446015, No. U1507115, and No. U1507118) and Natural Science Foundation of Jiangsu Province (No. BK20140534, No. BK20140580, No. BK20151350, and No. BK20131223).
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Zheng, X., Liu, E., Zhang, F. et al. Selective adsorption and separation of gadolinium with three-dimensionally interconnected macroporous imprinted chitosan films. Cellulose 24, 977–988 (2017). https://doi.org/10.1007/s10570-016-1136-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10570-016-1136-2