Abstract
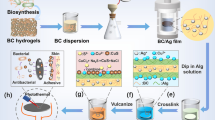
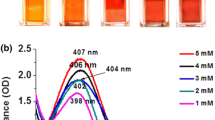
The increasing resistance of pathogens and bacteria is a serious problem in the medical treatment of wounds and injuries. Therefore, new therapeutic agents are not solely based on antibiotics, but also on the use of antimicrobial metal nanoparticles. In this paper we present an innovative method to prepare porous hybrids consisting of bacterial nanocellulose (BNC) and silver nanoparticles (AgNPs). The stepwise modification is based on fairly simple chemical reactions already described for two-dimensional cellulose films. We transferred this method to the three-dimensional, porous network of BNC leading to an antimicrobial activation of its surface. Compared to former approaches, the ultrafine network structure of BNC is less damaged by using mild chemicals. The amount and distribution of the AgNPs on the modified BNC was investigated using scanning electron microscopy. The AgNPs are firmly immobilized on the top and bottom surface of the BNC by chemical interactions. Their size and quantity increase with an increasing concentration of AgNO3 and extended reaction time in the AgNO3 solution. A strong antimicrobial activity of the BNC-AgNP hybrids against Escherichia coli was detected. Furthermore, agar diffusion tests confirmed that this activity is restricted to the modified dressing itself, avoiding a release of NPs into the wound. Therefore, the produced hybrids could be potentially suited as novel antimicrobial wound dressings.







Similar content being viewed by others
References
Alila S, Ferraria AM, Botelho do Rego AM, Boufi S (2009) Controlled surface modification of cellulose fibers by amino derivatives using N N’-carbonyldiimidazole as activator. Carbohydr Polym 77(3):553–562. doi:10.1016/j.carbpol.2009.01.028
Arora A, Mitragotri S (2010) Novel topical microbicides through combinatorial strategies. Pharm Res 27(7):1264–1272. doi:10.1007/s11095-010-0095-9
Barud HS, Regiani T, Marques RFC, Lustri WR, Messaddeq Y, Ribeiro SJL (2011) Antimicrobial bacterial cellulose—silver nanoparticles composite membranes. J Nanomat 2011:1–8. doi:10.1155/2011/721631
Bast E (2001) Mikrobiologische Methoden: Eine Einführung in grundlegende Arbeitstechniken, 2nd edn. Spektrum Akademischer Verlag GmbH, Heidelberg
Bhanat K, Parashar N, Jain K, Sharma VK (2011) Synthesis and antimicrobial study of 4-Benzylidene-2-phenyl-1-(5-phenylthiazole-2-yl)-1H-imidazol-5(4H)-one. Asian J Biochem Pharm Res 1(1):83–90
Boufi S, Ferraria AM, Botelho do Rego AM, Battaglini N, Herbst F, Rei Vilar M (2011) Surface functionalization of cellulose with noble metals nanoparticles through a selective nucleation. Carbohydr Polym 86(4):1586–1594. doi:10.1016/j.carbpol.2011.06.067
Bridges K, Kidson A, Lowbury EJL, Wilkins MS (1979) Gentamicin- and silver-resistant pseudomonas in a burns unit. Br Med J 1(6161):446–449. doi:10.1136/bmj.1.6161.446
Calfee DP (2012) Crisis in hospital-acquired, healthcare-associated infections. Annu Rev Med 63:359–371. doi:10.1146/annurev-med-081210-144458
Castellano JJ, Shafii SM, Ko F, Donate G, Wright TE, Mannari RJ, Payne WG, Smith DJ, Robson MC (2007) Comparative evaluation of silver-containing antimicrobial dressings and drugs. Int Wound J 4(2):114–122. doi:10.1111/j.1742-481X.2007.00358_2.x
Chawla A, Sharma A, Sharma AK (2012) A convenient approach for the synthesis of imidazole derivatives using microwaves. Chem Inform 43(24):116–140. doi:10.1002/chin.201224254
Cho K-H, Park J-E, Osaka T, Park S-G (2005) The study of antimicrobial activity and preservative effects of nanosilver ingredient. Electrochim Acta 51(5):956–960. doi:10.1016/j.electacta.2005.04.071
Chopra I (2007) The increasing use of silver-based products as antimicrobial agents: a useful development or a cause for concern? J Antimicrob Chemother 59(4):587–590. doi:10.1093/jac/dkm006
Czaja W, Krystynowicz A, Bielecki S, Brown RM Jr (2006) Microbial cellulose—the natural power to heal wounds. Biomaterials 27(2):145–151. doi:10.1016/j.biomaterials.2005.07.035
Czaja WK, Young DJ, Kawecki M, Brown RM Jr (2007) The future prospects of microbial cellulose in biomedical applications. Biomacromolecules 8(1):1–12. doi:10.1021/bm060620d
Ferraria AM, Boufi S, Battaglini N, Botelho do Rego AM, ReiVilar M (2010) Hybrid systems of silver nanoparticles generated on cellulose surfaces. Langmuir 26(3):1996–2001. doi:10.1021/la902477q
Fontana JD, De Souza AM, Fontana CK, Torriani IL, Moreschi JC, Gallotti BJ, De Souza SJ, Narcisco GP, Bichara JA, Farah LFX (1990) Acetobacter cellulose pellicle as a temporary skin substitute. Appl Biochem Biotechnol 24–25(1):253–264. doi:10.1007/BF02920250
Gallant-Behm CL, Yin HQ, Liu S, Heggers JP, Langford RE, Olson ME, Hart DA, Burrell RE (2005) Comparison of in vitro disc diffusion and time kill-kinetic assays for the evaluation of antimicrobial wound dressing efficacy. Wound Repair Regen 13(4):412–421. doi:10.1111/j.1067-1927.2005.130409.x
Gordon O, Slenters TV, Brunetto PS, Villaruz AE, Sturdevant DE, Otto M, Landmann R, Fromm KM (2010) Silver coordination polymers for prevention of implant infection: Thiol interaction, Impact on respiratory chain enzymes, and Hydroxyl Radical induction. Antimicrob Agents Chemother 54(10):4208–4218. doi:10.1128/AAC.01830-09
Heinze T, Liebert T, Heublein B, Hornig S (2006) Functional Polymers Based on Dextran. In: Klemm D (ed) Polysaccharides II Advances in Polymer Science, vol. 205, Springer, Heidelberg, pp 199–291. doi: 10.1007/12_100
Hestrin S, Schramm M (1954) Synthesis of cellulose by Acetobacter xylinum. 2. Preparation of freeze-dried cells capable of polymerizing glucose to cellulose. Biochem J 58(2):345–352
Hipler U-C, Elsner P, Fluhr JW (2006) Antifungal and antibacterial properties of a silver-loaded cellulosic fiber. J Biomed Mater Res B Appl Biomater 77B(1):156–163. doi:10.1002/jbm.b.30413
Ishida O, Kim D-Y, Kuga S, Nishiyama Y, Brown RM Jr (2004) Microfibrillar carbon from native cellulose. Cellulose 11(3–4):475–480. doi:10.1023/B:CELL.0000046410.31007.0b
Jonas R, Farah LF (1998) Production and application of microbial cellulose. Polym Degrad Stab 59(1–3):101–106. doi:10.1016/S0141-3910(97)00197-3
Klemm D, Schumann D, Udhardt U, Marsch S (2001) Bacterial synthesized cellulose—artificial blood vessels for microsurgery. Prog Polym Sci 26(9):1561–1603. doi:10.1016/S0079-6700(01)00021-1
Klemm D, Heublein B, Fink H-P, Bohn A (2005) Cellulose: fascinating biopolymer and sustainable Raw material. Angew Chem Int Edit 44(22):3358–3393. doi:10.1002/anie.200460587
Klemm D, Schumann D, Kramer F, Heßler N, Hornung M, Schmauder H-P, Marsch S (2006) Nanocelluloses as innovative polymers in research and application. Adv Polym Sci 205(August):49–96. doi:10.1007/12_097
Klemm D, Kramer F, Moritz S, Lindström T, Ankerfors M, Gray D, Dorris A (2011) Nanocelluloses: a new family of nature-based materials. Angew Chem Int Edit 50(24):5438–5466. doi:10.1002/anie.201001273
Knetsch MLW, Koole LH (2011) New strategies in the development of antimicrobial coatings: the example of increasing usage of silver and silver nanoparticles. Polymers 3(1):340–366. doi:10.3390/polym3010340
Kralisch D, Hessler N, Klemm D, Erdmann R, Schmidt W (2010) White biotechnology for cellulose manufacturing—the HoLiR concept. Biotechnol Bioeng 105(4):740–747. doi:10.1002/bit.22579
Loh JV, Percival SL, Woods EJ, Williams NJ, Cochrane CA (2009) Silver resistance in MRSA isolated from wound and nasal sources in humans and animals. Int Wound J 6(1):32–38. doi:10.1111/j.1742-481X.2008.00563.x
Maneerung T, Tokura S, Rujiravanit R (2008) Impregnation of silver nanoparticles into bacterial cellulose for antimicrobial wound dressing. Carbohydr Polym 72(1):43–51. doi:10.1016/j.carbpol.2007.07.025
Monteiro DR, Gorup LF, Takamiya AS, Ruvollo-Filho AC, de Camargo ER, Barbosa DB (2009) The growing importance of materials that prevent microbial adhesion: antimicrobial effect of medical devices containing silver. Int J Antimicrob Agents 34(2):103–110. doi:10.1016/j.ijantimicag.2009.01.017
Morones JR, Elechiguerra JL, Camacho A, Holt K, Kouri JB, Ramírez JT, Yacaman MJ (2005) The bactericidal effect of silver nanoparticles. Nanotechnology 16(10):2346–2353. doi:10.1088/0957-4484/16/10/059
Panyala NR, Peña-Méndez EM, Havel J (2008) Silver or silver nanoparticles: a hazardous threat to the environment and human health? J Appl Biomed 6(3):117–129
Percival SL, Bowler PG, Russell D (2005) Bacterial resistance to silver in wound care. J Hosp Infect 60(1):1–7. doi:10.1016/j.jhin.2004.11.014
Rodríguez-Gattorno G, Díaz D, Rendón L, Hernández-Segura GO (2002) Metallic Nanoparticles from Spontaneous Reduction of Silver(I) in DMSO. Interaction between Nitric Oxide and Silver Nanoparticles. J Phys Chem B 106(10):2482–2487. doi:10.1021/jp012670c
Schierholz JM, Lucas LJ, Rump A, Pulverer G (1998) Efficacy of silver-coated medical devices. J Hosp Infect 40(1):257–262. doi:10.1016/S0195-6701(98)90301-2
Silver S (2003) Bacterial silver resistance: molecular biology and uses and misuses of silver compounds. FEMS Microbiol Rev 27(2–3):341–353. doi:10.1016/S0168-6445(03)00047-0
Solway DR, Clark WA, Levinson DJ (2011) A parallel open-label trial to evaluate microbial cellulose wound dressing in the treatment of diabetic foot ulcers. Int Wound J 8(1):69–73. doi:10.1111/j.1742-481X.2010.00750.x
Sondi I, Salopek-Sondi B (2004) Silver nanoparticles as antimicrobial agent: a case study on E. coli as a model for Gram-negative bacteria. J Colloid Interface Sci 275(1):177–182. doi:10.1016/j.jcis.2004.02.012
Sureshkumar M, Siswanto DY, Lee C-K (2010) Magnetic antimicrobial nanocomposite based on bacterial cellulose and silver nanoparticles. J Mater Chem 20(33):6948–6955. doi:10.1039/c0jm00565g
Walker M, Hobot JA, Newman GR, Bowler PG (2003) Scanning electron microscopic examination of bacterial immobilisation in a carboxymethyl cellulose (AQUACEL) and alginate dressings. Biomaterials 24(5):883–890
Waring MJ, Parsons D (2001) Physico-chemical characterization of carboxymethylated spun cellulose fibres. Biomaterials 22(9):903–912. doi:10.1016/S0142-9612(02)00414-3
Wesarg F, Schlott F, Grabow J, Kurland H-D, Heßler N, Kralisch D, Müller FA (2012) In situ synthesis of photocatalytically active hybrids consisting of bacterial nanocellulose and anatase nanoparticles. Langmuir 28(37):13518–13525. doi:10.1021/la302787z
Wiegand C, Elsner P, Hipler U-C, Klemm D (2006) Protease and ROS activities influenced by a composite of bacterial cellulose and collagen type I in vitro. Cellulose 13(6):689–696. doi:10.1007/s10570-006-9073-0
Yang J, Yu J, Sun D, Yang X (2011) Preparation of novel Ag/bacterial cellulose hybrid nanofibers for antimicrobial wound dressing. Adv Mat Res 152-153:1771-1774. doi: 10.4028/www.scientific.net/AMR.152-153.1771
Yang G, Xie J, Hong F, Cao Z, Yang X (2012) Antimicrobial activity of silver nanoparticle impregnated bacterial cellulose membrane: effect of fermentation carbon sources of bacterial cellulose. Carbohydr Polym 87(1):839–845. doi:10.1016/j.carbpol.2011.08.079
Yun YS, Bak H, Jin H-J (2010) Monolithic macroporous carbon cryogel prepared from natural polymers. J Korean Phys Soc 57(6):1950–1952. doi:10.3938/jkps.57.1950
Zimmermann R, Pfuch A, Horn K, Weisser J, Heft A, Röder M, Linke R, Schnabelrauch M, Schimanski A (2011) An approach to create silver containing antimicrobial coatings by use of atmospheric pressure plasma chemical vapour deposition (APCVD) and combustion chemical vapour deposition (CCVD) in an economic way. Plasma Process Polym 8(4):295–304. doi:10.1002/ppap.201000113
Acknowledgments
The authors thank R. Zimmermann, INNOVENT e.V. Technologieentwicklung Jena, for his kind support during the antimicrobial studies and D. Reichmann for agar diffusion tests (Department of Dermatology and Allergology). F.W. acknowledges the DBU (Deutsche Bundesstiftung Umwelt) for financial support. C.W., D.K. and F.A.M. are grateful for the funding of this work by the Thuringian Ministry of Education, Science and Culture (B714-10032) and the European Fund for Regional Development.
Author information
Authors and Affiliations
Corresponding author
Additional information
S. Berndt and F. Wesarg contributed equally to this work. D. Kralisch and F. A. Müller are members of the Jena Center for Soft Matter.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Berndt, S., Wesarg, F., Wiegand, C. et al. Antimicrobial porous hybrids consisting of bacterial nanocellulose and silver nanoparticles. Cellulose 20, 771–783 (2013). https://doi.org/10.1007/s10570-013-9870-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10570-013-9870-1