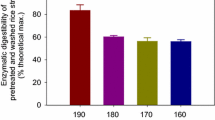
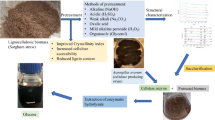
Abstract
Wheat straw is an abundant residue of agriculture which is increasingly considered as a feedstock for the production of fuels, energy and chemicals. The concentrated acid hydrolysis of wheat straw has been investigated in this work. Hemicellulose and cellulose have been efficiently converted into monomers of pentoses and glucose in high yields by a one-pot decrystallization-hydrolysis procedure. This process differs from usual concentrated acid biomass fractionation methodologies as a low quantity of acid is used and the supplementary use of a costly acid is not necessary to yield efficiently carbohydrates. The influence of the acid native concentration, and of the time of the decrystallization step have been studied so as to optimise yields of carbohydrates using a minimum of sulfuric acid so as to preserve a potential market value of the process. One can also imagine that this procedure will not impact dramatically the subsequent purification costs. In view of the growing importance of renewable resource-based molecules in the chemical industry, and the necessity to produce fermentable substrate for biofuels, this approach may open a new avenue for the use of wheat straw as raw material for various applications.





Similar content being viewed by others
References
Cara C, Moya M, Ballesteros I, Negro MJ, González A, Ruiz E (2007) Influence of solid loading on enzymatic hydrolysis of steam exploded or liquid hot water pretreated olive tree biomass. Proc Biochem 42:1003–1009
Harmer MA, Fan A, Liauw A, Kumar RK (2009) A new route to high yield sugars from biomass: phosphoric–sulfuric acid. Chem commun 43:6610–6612
Jayme G, Lang F (1963) Cellulose solvents. Methods Carbohydr Chem 3:75–83
Kamm B, Kamm M, Schmidt M, Hirth T, Schulze M (2006) Lignocellulose-based chemical products and product family trees. In: Kamm B, Gruber PR, Kamm M (eds) Biorefineries—industrial processes and products, vol. 2, Wiley-VCH, Weinheim
Klass DL (1998) Biomass for renewable energy, fuels, and chemicals. Academic press, San Diego
Lou J, Dawson KA, Strobel HJ (1997) Cellobiose and cellodextrin metabolism by the ruminal bacterium Ruminococcus albus. Curr Microbiol 35:221–227
Martel F, Estrine B, Plantier-Royon R, Hoffmann N, Portella C (2010) Development of agriculture left-overs: fine organic chemicals from wheat hemicellulose-derived pentoses. Top Curr Chem 294:79–115
Miller GL (1963) Cellodextrins. Methods Carbohydr Chem 3:134–139
Moxley G, Zhang Y-HP (2007) More accurate determination of acid-labile carbohydrate composition in lignocellulose by modified quantitative saccharification. Energy Fuels 21:3684–3688
Pereira AN, Mobedshahi M, Ladisch MR (1988) Preparation of cellodextrins. Methods Enzymol 160:26–43
Rinaldi R (2009) Acid hydrolysis of cellulose as the entry point into biorefinery schemes. ChemSusChem 12:1096–1107
Ritter GJ, Mitchell RL, Seborg RM (1933) Some factors that influence the conversion of cellulosic materials to sugar. J Am Chem Soc 55:2989–2991
Rollin JA, Zhu Z, Sathisuksanoh N, Zhang Y-HP (2011) Increasing cellulose accessibility is more important than removing lignin: a comparison of cellulose solvent-based lignocellulose fractionation and soaking in aqueous ammonia. Biotechnol Bioeng 108:22–30
Sathitsuksanoh N, Zhu ZG, Wi S, Zhang Y-HP (2010) Cellulose solvent-based biomass pretreatment breaks highly ordered hydrogen bonds in cellulose fibers of switchgrass. Biotechnol Bioeng 108:521–529
Sluiter A, Hames B, Ruiz R, Scarlata C, Sluiter J, Templeton D, Crocker D (2007) Determination of structural carbohydrates and lignin in biomass, laboratory analytical procedure-002, 003, 017, 019. National Renewable Energy Laboratory, U.S. Department of Energy, USA
Zhang Y-HP, Lynd LR (2003) Cellodextrin preparation by mixed-acid hydrolysis and chromatographic separation. Anal Biochem 322:225–232
Zhang Y-HP, Cui J, Lynd LR, Kuang LR (2006) A transition from cellulose swelling to cellulose dissolution by o-phosphoric acid: evidence from enzymatic hydrolysis and supramolecular structure. Biomacromolecules 7:644–648
Zhang Y-HP, Ding S-Y, Mielenz JR, Elander R, Laser M, Himmel ME, McMillan JD, Lynd LR (2007) Fractionating recalcitrant lignocellulose at modest reaction conditions. Biotechnol Bioeng 97:214–223
Zhu Z, Sathitsuksanoh N, Vinzant T, Schell DJ, McMillan JD, Zhang Y-HP (2009) Comparative study of corn stover pretreated by dilute acid and cellulose solvent-based lignocellulose fractionation: enzymatic hydrolysis, supramolecular structure, and substrate accessibility. Biotechnol Bioeng 103:715–724
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Hausser, N., Marinkovic, S. & Estrine, B. Improved sulfuric acid decrystallization of wheat straw to obtain high yield carbohydrates. Cellulose 18, 1521–1525 (2011). https://doi.org/10.1007/s10570-011-9599-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10570-011-9599-7