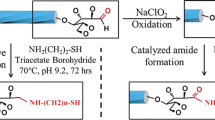
Abstract
Cellulose triacetate (CTA) derivatives having a disulfide group at the reducing-end (CTA2S, CTA13S, CTA41S), with number average degrees of polymerization (DPns) of 2, 13 and 41, respectively, were prepared. The CTA-self-assembled gold nanoparticles (CTA2Au, CTA13Au, and CTA41Au) were obtained through the reduction of gold salt (HAuCl4) with CTASs. The diameters (d) and the interparticle distances (L) of the gold cores were analyzed by transmission electron microscopy (TEM) observations. The d values of CTA2Au, CTA13Au, and CTA41Au, were 8.7, 7.9, and 13.4 nm respectively. The L values of CTA2Au, CTA13Au, and CTA41Au, were 2.8, 6.3, and 20.9 nm, respectively, and agreed well with the molecular length (l) of CTAS chains (ls of CTA2S, CTA13S, CTA41S = 2.0, 7.5, 21.5 nm, respectively). The hydrodynamic diameters (D) of CTAAu nanoparticles in chloroform solution, measured by dynamic light scattering (DLS), were larger than the d values and increased with the increase in the molecular length of the CTA chains. The CTAS chain was found to work as an excellent stabilizer of the gold nanoparticles in both solid state and solution. The molecular length of CTA chains controlled the size and the alignment of the gold nanoparticles. As a result, the radially oriented CTA chains on the gold nanoparticles were successfully prepared.








Similar content being viewed by others
References
Arndt P, Bockholt K, Gerdes R, Huschens S, Pyplo J, Redlich H, Samm K (2003) Cellulose oligomers: preparation from cellulose triacetate, chemical transformations and reactions. Cellulose 10:75–83
Arndt P, Gerdes R, Huschens S, Pyplo-Schnieders J, Redlich H (2005) Preparation of cellulose oligomers from cellulose triacetate (standard procedure). Cellulose 12:317–326
Atalla RH, Ellia JD, Schroeder LR (1984) Some eeffects of elevated temperatures on the structure of cellulose and its transformation. J Wood Chem Technol 4:465–482
Azzam T, Eisenberg A (2007) Monolayer-protected gold nanoparticles by the self-assembly of micellar poly (ethylene oxide)-b-poly(epsilon-caprolactone) block copolymer. Langmuir 23:2126–2132
Bernet B, Xu JW, Vasella A (2000) Oligosaccharide analogues of polysaccharides part 20—NMR analysis of templated cellodextrins possessing two parallel chains: A mimic for cellulose I. Helv Chim Acta 83:2072–2114
Brust M, Kiely CJ (2002) Some recent advances in nanostructure preparation from gold and silver particles: A short topical review. Colloids Surf A-Physicochem Eng Aspects 202:175–186
Brust, M., Walker, M., Bethell, D., Schiffrin, D.J. and Whyman, R. (1994) Synthesis of thiol-derivatized gold nanoparticles in a 2-phase liquid-liquid system. Journal of the Chemical Society-Chemical Communications 801–802
Brust, M., Fink, J., Bethell, D., Schiffrin, D.J. and Kiely, C. (1995) Synthesis and reactions of functionalized gold nanoparticles. Journal of the Chemical Society-Chemical Communications 1655–1656
Ceresa RJ (1961) The synthesis of block and graft copolymers of cellulose and its derivatives. Polymer 2:213–219
Corbierre MK, Cameron NS, Lennox RB (2004) Polymer-stabilized gold nanoparticles with high grafting densities. Langmuir 20:2867–2873
Daniel MC, Astruc D (2004) Gold nanoparticles: Assembly, supramolecular chemistry, quantum-size-related properties, and applications toward biology, catalysis, and nanotechnology. Chem Rev 104:293–346
de Oliveira W, Glasser WG (1994a) Multiphase materials with lignin. 13. Block-copolymers with cellulose propionate. Polymer 35:1977–1985
de Oliveira W, Glasser WG (1994b) Novel cellulose derivatives. 2. Synthesis and characteristics of mono-functional cellulose propionate segments. Cellulose 1:77–86
Dulmage WJ (1957) The molecular and crystal structure of cellulose triacetate. J Polym Sci 26:277–288
Edgar KJ, Buchanan CM, Debenham JS, Rundquist PA, Seiler BD, Shelton MC, Tindall D (2001) Advances in cellulose ester performance and application. Prog Polym Sci 26:1605–1688
Enomoto Y, Kamitakahara H, Takano T, Nakatsubo F (2006) Synthesis of diblock copolymers with cellulose derivatives. 3. Cellulose derivatives carrying a single pyrene group at the reducing-end and fluorescent studies of their self-assembly systems in aqueous NaoH solutions. Cellulose 13:437–448
Enomoto-Rogers Y, Kamitakahara H, Nakayama K, Takano T, Nakatsubo F (2009a) Synthesis and thermal properties of poly(methyl methacrylate)-graft-(cellobiosylamine-C15). Cellulose 16:519–530
Enomoto-Rogers Y, Kamitakahara H, Takano T, Nakatsubo F (2009b) Cellulosic graft copolymer: Poly(methyl methacrylate) with cellulose side chains. Biomacromolecules 10:2110–2117
Feger C, Cantow HJ (1980) Cellulose containing block co-polymers. 1. Synthesis of trimethylcellulose-(b-poly(oxytetramethylene))-star block co-polymers. Polym Bull 3:407–413
Fort RJ, Hutchinson RJ, Moore WR, Murphy M (1963) Viscosity temperature relationships for dilute solutions of high polymers. Polymer 4:35–46
Glasser WG (2004) Prospects for future applications of cellulose acetate. Macromol Symp 208:371–394
Gulari E, Gulari E, Tsunashima Y, Chu B (1979) Photon-correlation spectroscopy of particle distributions. J Chem Phys 70:3965–3972
Haruta M, Date M (2001) Advances in the catalysis of au nanoparticles. Appl Catal A-Gen 222:427–437
Heath JR, Knobler CM, Leff DV (1997) Pressure/temperature phase diagrams and superlattices of organically functionalized metal nanocrystal monolayers: the influence of particle size, size distribution, and surface passivant. J Phys Chem B 101:189–197
Howard P, Parikh RS (1968) Solution properties of cellulose triacetate. 2. Solubility and viscosity studies. J Polym Sci A Polym Chem 6:537–546
Isogai A, Usuda M (1991) Preparation of low-molecular-weight celluloses using phosphoric-acid. Mokuzai Gakkaishi 37:339–344
Kamitakahara H, Nakatsubo F (2005) Synthesis of diblock copolymers with cellulose derivatives. 1. Model study with azidoalkyl carboxylic acid and cellobiosylamine derivative. Cellulose 12:209–219
Kamitakahara H, Enomoto Y, Hasegawa C, Nakatsubo F (2005) Synthesis of diblock copolymers with cellulose derivatives. 2. Characterization and thermal properties of cellulose triacetate-block-oligoamide-15. Cellulose 12:527–541
Katz E, Willner I (2004) Integrated nanoparticle-biomolecule hybrid systems: synthesis, properties, and applications. Angew Chem-Int Ed 43:6042–6108
Kim S, Stannett VT, Gilbert RD (1973) New class of biodegradable polymers. J Polym Sci C-Polym Lett 11:731–735
Kim S, Stannett VT, Gilbert RD (1976) Biodegradable cellulose block copolymers. J Macromol Sci-Chem A 10:671–679
Kolpak FJ, Blackwell J (1976) Determination of structure of cellulose II. Macromolecules 9:273–278
Kono H, Numata Y, Nagai N, Erata T, Takai M (1999) Studies of the series of cellooligosaccharide peracetates as a model for cellulose triacetate by 13C CP/MAS NMR spectroscopy and x-ray analyses. Carbohydr Res 322:256–263
Kono H, Erata T, Takai M (2002) CP/MAS 13C NMR study of cellulose and cellulose derivatives. 2. Complete assignment of the 13C resonance for the ring carbons of cellulose triacetate polymorphs. J Am Chem Soc 124:7512–7518
Kumar R, Pandey AK, Tyagi AK, Dey GK, Ramagiri SV, Bellare JR, Goswami A (2009) In situ formation of stable gold nanoparticles in polymer inclusion membranes. J Colloid Interf Sci 337:523–530
Lai MK, Chang CY, Lien YW, Tsiang RCC (2006) Application of gold nanoparticles to microencapsulation of thioridazine. J Control Release 111:352–361
Li ZH, Taubert A (2009) Cellulose/gold nanocrystal hybrids via an ionic liquid/aqueous precipitation route. Molecules 14:4682–4688
Li DX, Cui Y, Wang KW, He Q, Yan XH, Li JB (2007a) Thermosensitive nanostructures comprising gold nanoparticles grafted with block copolymers. Adv Funct Mater 17:3134–3140
Li DX, He Q, Cui Y, Wang KW, Zhang XM, Li JB (2007b) Thermosensitive copolymer networks modify gold nanoparticles for nanocomposite entrapment. Chem-A Eur J 13:2224–2229
Link S, El-Sayed MA (1999) Size and temperature dependence of the plasmon absorption of colloidal gold nanoparticles. J Phys Chem B 103:4212–4217
Liu Z.M, Li M, Turyanska L, Makarovsky O, Patane A, Wu WJ, Mann S (2010) Self-assembly of electrically conducting biopolymer thin films by cellulose regeneration in gold nanoparticle aqueous dispersions. Chemistry of Materials 22:2675–2680
Loskutov AI, Uryupina OY, Vysotskii VV, Roldughin VI (2009) Surface faceting of gold nanoparticles and adsorption of organic macromolecules. Colloid J 71:668–671
Maye MM, Lou YB, Zhong CJ (2000) Core-shell gold nanoparticle assembly as novel electrocatalyst of co oxidation. Langmuir 16:7520–7523
Mezger T, Cantow HJ (1983a) Cellulose containing block co-polymers. 4. Cellulose triester macroinitiators. Angew Makromol Chem 116:13–27
Mezger T, Cantow HJ (1983b) Cellulose containing block co-polymers. 5. Threeblock co-polymer syntheses via macroinitiator. Makromol Chem-Rapid Commun 4:313–320
Mezger T, Cantow HJ (1984) Cellulose-containing triblock copolymers—syntheses via cellulosic dithiodiaryl photoinitiators. Polym Photochem 5:49–56
Murty KVSN, Xie T, Bernet B, Vasella A (2006) Oligosaccharide analogues of polysaccharides—part 26—mimics of cellulose I and cellulose II: Di- and monoalkynyl c-cellosides of 1, 8-disubstituted anthraquinones. Helv Chim Acta 89:675–730
Nakatsubo F, Maeda K, Murakami K (1987) Reactivity of the reducing-end of cellulose. 1. Preparation of phenylcelluloside. Bull Kyoto Univ Forests 59:301
Ohno K, Koh K, Tsujii Y, Fukuda T (2002) Synthesis of gold nanoparticles coated with well-defined, high-density polymer brushes by surface-initiated living radical polymerization. Macromolecules 35:8989–8993
Pinto RJB, Marques P, Martins MA, Neto CP, Trindade T (2007) Electrostatic assembly and growth of gold nanoparticles in cellulosic fibres. J Colloid Interf Sci 312:506–512
Pohjola L, Eklund V (1977) Polyurethane block copolymers from cellulose triacetate. Paperi Ja Puu-Paper And Timber 59:117–120
Roche EJ, Obrien JP, Allen SR (1986) Preparation of cellulose triacetate: From solution. Polym Commun 27:138–140
Sata H, Murayama M, Shimamoto S (2004) Properties and applications of cellulose triacetate film. Macromol Symp 208:323–333
Schubert MM, Hackenberg S, van Veen AC, Muhler M, Plzak V, Behm RJ (2001) Co oxidation over supported gold catalysts-“inert” and “active” support materials and their role for the oxygen supply during reaction. J Catal 197:113–122
Shin Y, Bae IT, Arey BW, Exarhos GJ (2008) Facile stabilization of gold-silver alloy nanoparticles on cellulose nanocrystal. J Phys Chem C 112:4844–4848
Sipahi-Saglam E, Gelbrich M, Gruber E (2003) Topochemically modified cellulose. Cellulose 10:237–250
Sprague BS, Riley JL, Noether HD (1958) Factors influencing the crystal structure of cellulose triacetate. Text Res J 28:275–287
Stannett VT, Williams JL (1976) Modification of wool and cellulose fibers by grafting. J Macromol Sci-Chem A 10:637–652
Steinmann HW (1970) Elastomeric fibers from cellulose triacetate. Polym prepr Am Chem Soc. Div Polym Chem 11:285–290
Stipanovic AJ, Sarko A (1978) Molecular and crystal-structure of cellulose triacetate: - parallel chain structure. Polymer 19:3–8
Strong L, Whitesides GM (1988) Structures of self-assembled monolayer films of organosulfur compounds adsorbed on gold single-crystals—electron-diffraction studies. Langmuir 4:546–558
Sugiyama J, Vuong R, Chanzy H (1991) Electron-diffraction study on the 2 crystalline phases occurring in native cellulose from an algal cell-wall. Macromolecules 24:4168–4175
Wenz G, Liepold P, Bordeanu N (2004) Monolayers of reactive cellulose derivatives. Macromol Symp 210:203–208
Wenz G, Liepold P, Bordeanu N (2005) Synthesis and sam formation of water soluble functional carboxymethylcelluloses: Thiostilfates and thioethers. Cellulose 12:85–96
Woodcock C, Sarko A (1980) Packing analysis of carbohydrates and polysaccharides.11. Molecular and crystal-structure of native ramie cellulose. Macromolecules 13:1183–1187
Wuelfing WP, Gross SM, Miles DT, Murray RW (1998) Nanometer gold clusters protected by surface-bound monolayers of thiolated poly(ethylene glycol) polymer electrolyte. J Am Chem Soc 120:12696–12697
Yagi S, Kasuya N, Fukuda K (2010) Synthesis and characterization of cellulose-b-polystyrene. Polymer Journal 42:342–348
Yokota S, Kitaoka T, Opietnik M, Rosenau T, Wariishi H (2008) Synthesis of gold nanoparticles for in situ conjugation with structural carbohydrates. Angew Chem Int Ed 47:9866–9869
Yonezawa T, Onoue S, Kimizuka N (2001a) Formation of uniform fluorinated gold nanoparticles and their highly ordered hexagonally packed monolayer. Langmuir 17:2291–2293
Yonezawa T, Yasui K, Kimizuka N (2001b) Controlled formation of smaller gold nanoparticles by the use of four-chained disulfide stabilizer. Langmuir 17:271–273
Acknowledgments
This study was supported in part by a Grand-in-Aid from a Research Fellowships of the Japan Society for the Promotion of Science (JSPS) for Young Scientists (Y.E-R), and by a Grant-in-Aid for Scientific Research from the Ministry of Education, Science, and Culture of Japan (Nos. 18688009 and 21580205).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Enomoto-Rogers, Y., Kamitakahara, H., Yoshinaga, A. et al. Radially oriented cellulose triacetate chains on gold nanoparticles. Cellulose 17, 923–936 (2010). https://doi.org/10.1007/s10570-010-9437-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10570-010-9437-3