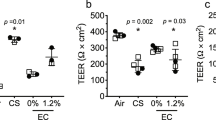
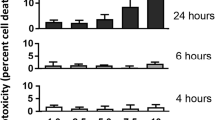
Abstract
Smoke inhalation injury is the leading cause of death in firefighters and victims. Inhaled hot air and toxic smoke are the predominant hazards to the respiratory epithelium. We aimed to analyze the effects of thermal stress and smoke aldehyde on the permeability of the airway epithelial barrier. Transepithelial resistance (RTE) and short-circuit current (ISC) of mouse tracheal epithelial monolayers were digitized by an Ussing chamber setup. Zonula occludens-1 tight junctions were visualized under confocal microscopy. A cell viability test and fluorescein isothiocyanate-dextran assay were performed. Thermal stress (40 °C) decreased RTE in a two-phase manner. Meanwhile, thermal stress increased ISC followed by its decline. Na+ depletion, amiloride (an inhibitor for epithelial Na+ channels [ENaCs]), ouabain (a blocker for Na+/K+-ATPase), and CFTRinh-172 (a blocker of cystic fibrosis transmembrane regulator [CFTR]) altered the responses of RTE and ISC to thermal stress. Steady-state 40 °C increased activity of ENaCs, Na+/K+-ATPase, and CFTR. Acrolein, one of the main oxidative unsaturated aldehydes in fire smoke, eliminated RTE and ISC. Na+ depletion, amiloride, ouabain, and CFTRinh-172 suppressed acrolein-sensitive ISC, but showed activating effects on acrolein-sensitive RTE. Thermal stress or acrolein disrupted zonula occludens-1 tight junctions, increased fluorescein isothiocyanate-dextran permeability but did not cause cell death or detachment. The synergistic effects of thermal stress and acrolein exacerbated the damage to monolayers. In conclusion, the paracellular pathway mediated by the tight junctions and the transcellular pathway mediated by active and passive ion transport pathways contribute to impairment of the airway epithelial barrier caused by thermal stress and acrolein.

Thermal stress and acrolein are two essential determinants for smoke inhalation injury, impairing airway epithelial barrier.
Transcellular ion transport pathways via the ENaC, CFTR, and Na/K-ATPase are interrupted by both thermal stress and acrolein, one of the most potent smoke toxins.
Heat and acrolein damage the integrity of the airway epithelium through suppressing and relocating the tight junctions.








Similar content being viewed by others
Abbreviations
- ENaCs:
-
Epithelial Na+ channels
- CFTR:
-
Cystic fibrosis transmembrane regulator
- R TE :
-
Transepithelial resistance
- I SC :
-
Short-circuit current
- MTE:
-
Mouse tracheal epithelial
- FITC:
-
Fluorescein isothiocyanate
- HBE:
-
Human bronchial epithelial
- DMSO:
-
Dimethyl sulfoxide
- ZO-1:
-
Zonula occludens-1
- P1:
-
Phase 1
- P2:
-
Phase 2
- ASI:
-
Amiloride-sensitive ISC
- KCs:
-
K+ channels
- ROS:
-
Reactive oxygen species
- CaCCs:
-
Ca2+-activated Cl− channels
- NKCC:
-
Na+/K+/2Cl
- MAPK:
-
Mitogen-activated protein kinase
References
Alexander NS, Blount A, Zhang S, Skinner D, Hicks SB, Chestnut M, et al. Cystic fibrosis transmembrane conductance regulator modulation by the tobacco smoke toxin acrolein. Laryngoscope. 2012;122:1193–7.
Alwis KU, de Castro BR, Morrow JC, Blount BC. Acrolein exposure in U.S. tobacco smokers and non-tobacco users: NHANES 2005-2006. Environ Health Perspect. 2015;123:1302–8.
Anthony TR, Joggerst P, James L, Burgess JL, Leonard SS, Shogren ES. Method development study for APR cartridge evaluation in fire overhaul exposures. Ann Occup Hyg. 2007;51:703–16.
Bein K, Leikauf GD. Acrolein - a pulmonary hazard. Mol Nutr Food Res. 2011;55:1342–60.
Borchers MT, Wert SE, Leikauf GD. Acrolein-induced MUC5ac expression in rat airways. Am J Phys. 1998;274:L573–81.
Burcham PC, Raso A, Thompson CA. Intermediate filament carbonylation during acute acrolein toxicity in A549 lung cells: functional consequences, chaperone redistribution, and protection by bisulfite. Antioxid Redox Signal. 2010;12(3):337–47.
Chang J, Ding Y, Zhou Z, Nie HG, Ji HL. Transepithelial fluid and salt re-absorption regulated by cGK2 signals. Int J Mol Sci. 2018;19:E881.
Chen Z, Zhao R, Zhao M, Liang X, Bhattarai D, Dhiman R, et al. Regulation of epithelial sodium channels in urokinase plasminogen activator deficiency. Am J Phys Lung Cell Mol Phys. 2014;307:L609–17.
Cui Y, Li H, Wu S, Zhao R, Du D, Ding Y, et al. Formaldehyde impairs transepithelial sodium transport. Sci Rep. 2016;6:35857.
Davidson DJ, Kilanowski FM, Randell SH, Sheppard DN, Dorin JR. A primary culture model of differentiated murine tracheal epithelium. Am J Phys Lung Cell Mol Phys. 2000;279(4):L766–78.
Dong ZW, Chen J, Ruan YC, Zhou T, Chen Y, Chen Y, et al. CFTR-regulated MAPK/NF-kappaB signaling in pulmonary inflammation in thermal inhalation injury. Sci Rep. 2015;5:15946.
Dubick MA, Carden SC, Jordan BS, Langlinais PC, Mozingo DW. Indices of antioxidant status in rats subjected to wood smoke inhalation and/or thermal injury. Toxicology. 2002;176(1–2):145–57.
Dunayevich P, Baltanas R, Clemente JA, Couto A, Sapochnik D, Vasen G, et al. Heat-stress triggers MAPK crosstalk to turn on the hyperosmotic response pathway. Sci Rep. 2018;8(1):15168.
Faroon O, Roney N, Taylor J, Ashizawa A, Lumpkin MH, Plewak DJ. Acrolein environmental levels and potential for human exposure. Toxicol Ind Health. 2008;24(8):543–64.
Farquhar MG, Palade GE. Junctional complexes in various epithelia. J Cell Biol. 1963;17:375–412. https://doi.org/10.1083/jcb.17.2.375.
Fitzgerald KT, Flood AA. Smoke inhalation. Clin Tech Small Anim Prac. 2006;21:205–14.
Flynn AN, Itani OA, Moninger TO, Welsh MJ. Acute regulation of tight junction ion selectivity in human airway epithelia. Proc Natl Acad Sci U S A. 2009;106:3591–6.
Fuller CM, Benos DJ. CFTR! Am J Phys. 1992;263:C267–86.
Gillie DJ, Pace AJ, Coakley RJ, Koller BH, Barker PM. Liquid and ion transport by fetal airway and lung epithelia of mice deficient in sodium-potassium-2-chloride transporter. Am J Respir Cell Mol Biol. 2001;25(1):14–20.
Hales CA, Barkin PW, Jung W, Trautman E, Lamborghini D, Herrig N, et al. Synthetic smoke with acrolein but not HCl produces pulmonary edema. J Appl Physiol (1985). 1988;64:1121–33.
Han DY, Nie HG, Gu X, Nayak RC, Su XF, Fu J, et al. K+ channel openers restore verapamil-inhibited lung fluid resolution and transepithelial ion transport. Respir Res. 2010;11:65.
Haponik EF. Clinical smoke inhalation injury: pulmonary effects. Occup Med. 1993;8:430–68.
Hermann A, Sitdikova GF, Weiger TM. Oxidative stress and maxi calcium-activated potassium (BK) channels. Biomolecules. 2015;5(3):1870–911.
Horani A, Dickinson JD, Brody SL. Applications of mouse airway epithelial cell culture for asthma research. Methods Mol Biol. 2013;1032:91–107.
Hou Y, Cui Y, Zhou Z, Liu H, Zhang H, Ding Y, et al. Upregulation of the WNK4 signaling pathway inhibits epithelial sodium channels of mouse tracheal epithelial cells after influenza A infection. Front Pharmacol. 2019;10:12.
Howard M, Roux J, Iles KE, Miyazawa B, Christiaans S, Anjum N, et al. Activation of the heat shock response attenuates the interleukin 1beta-mediated inhibition of the amiloride-sensitive alveolar epithelial ion transport. Shock. 2013;39:189–96.
Huang W, Zhao H, Dong H, Wu Y, Yao L, Zou F, et al. High-mobility group box 1 impairs airway epithelial barrier function through the activation of the RAGE/ERK pathway. Int J Mol Med. 2016;37(5):1189–98.
Kis A, Krick S, Baumlin N, Salathe M. Airway hydration, apical K(+) secretion, and the large-conductance, Ca(2+)-activated and voltage-dependent potassium (BK) channel. Ann Am Thorac Soc. 2016;13(Suppl 2):S163–8.
Kuninaka S, Ichinose Y, Koja K, Toh Y. Suppression of manganese superoxide dismutase augments sensitivity to radiation, hyperthermia and doxorubicin in colon cancer cell lines by inducing apoptosis. Br J Cancer. 2000;83(7):928–34.
Kuroishi S, Suda T, Fujisawa T, Ide K, Inui N, Nakamura Y, et al. Epithelial-mesenchymal transition induced by transforming growth factor-beta1 in mouse tracheal epithelial cells. Respirology. 2009;14:828–37.
Lee KE, Jee HM, Hong JY, Kim MN, Oh MS, Kim YS, et al. German cockroach extract induces matrix metalloproteinase-1 expression, leading to tight junction disruption in human airway epithelial cells. Yunsei Med J. 2018;59(10):1222–31.
Li JJ, Oberley LW. Overexpression of manganese-containing superoxide dismutase confers resistance to the cytotoxicity of tumor necrosis factor alpha and/or hyperthermia. Cancer Res. 1997;57(10):1991–8.
Li Y, Chang J, Cui Y, Zhao R, Ding Y, Hou Y, et al. Novel mechanisms for crotonaldehyde-induced lung edema. Oncotarget. 2017;8:83509–22.
Londino JD, Lazrak A, Collawn JF, Bebok Z, Harrod KS, Matalon S. Influenza virus infection alters ion channel function of airway and alveolar cells: mechanisms and physiological sequelae. Am J Phys Lung Cell Mol Phys. 2017;313(5):L845–l58.
Manzanares D, Gonzalez C, Ivonnet P, Chen RS, Valencia-Gattas M, Conner GE, et al. Functional apical large conductance, Ca2+-activated, and voltage-dependent K+ channels are required for maintenance of airway surface liquid volume. J Biol Chem. 2011;286(22):19830–9.
Matsuno T, Ito Y, Ohashi T, Morise M, Takeda N, Shimokata K, et al. Dual pathway activated by tert-butyl hydroperoxide in human airway anion secretion. J Pharmacol Exp Ther. 2008;327(2):453–64.
Meacher DM, Menzel DB. Glutathione depletion in lung cells by low-molecular-weight aldehydes. Cell Biol Toxicol. 1999;15:163–71.
Nie HG, Chen L, Han DY, Li J, Song WF, Wei SP, et al. Regulation of epithelial sodium channels by cGMP/PKGII. J Physiol. 2009;587:2663–76.
Omar RA, Yano S, Kikkawa Y. Antioxidant enzymes and survival of normal and simian virus 40-transformed mouse embryo cells after hyperthermia. Cancer Res. 1987;47(13):3473–6.
Reinhardt TE, Ottmar RD. Baseline measurements of smoke exposure among wildland firefighters. J Occup Environ Hyg. 2004;1:593–606.
Romet-Haddad S, Marano F, Blanquart C, Baeza-Squiban A. Tracheal epithelium in culture: a model for toxicity testing of inhaled molecules. Cell Biol Toxicol. 1992;8:141–50.
Roux E, Ouedraogo N, Hyvelin JM, Savineau JP, Marthan R. In vitro effect of air pollutants on human bronchi. Cell Biol Toxicol. 2002;18:289–99.
Sailland J, Grosche A, Baumlin N, Dennis JS, Schmid A, Krick S, et al. Role of Smad3 and p38 signalling in cigarette smoke-induced CFTR and BK dysfunction in primary human bronchial airway epithelial cells. Sci Rep. 2017;7(1):10506.
Schreiber R, Ousingsawat J. Regulation of TMEM16A/ANO1 and TMEM16F/ANO6 ion currents and phospholipid scrambling by Ca(2+) and plasma membrane lipid. J Physiol. 2018;596(2):217–29.
Scudieri P, Caci E, Venturini A, Sondo E, Pianigiani G, Marchetti C, et al. Ion channel and lipid scramblase activity associated with expression of TMEM16F/ANO6 isoforms. J Physiol. 2015;593(17):3829–48.
Sheppard DN, Welsh MJ. Structure and function of the CFTR chloride channel. Physiol Rev. 1999;79:S23–45.
Stevens JF, Maier CS. Acrolein: sources, metabolism, and biomolecular interactions relevant to human health and disease. Mol Nutr Food Res. 2008;52:7–25.
Tobey NA, Sikka D, Marten E, Caymaz-Bor C, Hosseini SS, Orlando RC. Effect of heat stress on rabbit esophageal epithelium. Am J Phys. 1999;276:G1322–30.
Tsukita S, Furuse M. Claudin-based barrier in simple and stratified cellular sheets. Curr Opin Cell Biol. 2002;14:531–6.
Umeda K, Ikenouchi J, Katahira-Tayama S, Furuse K, Sasaki H, Nakayama M, et al. ZO-1 and ZO-2 independently determine where claudins are polymerized in tight-junction strand formation. Cell. 2006;126:741–54.
Van Itallie CM, Anderson JM. Claudins and epithelial paracellular transport. Annu Rev Physiol. 2006;68:403–29.
Wang X, Adler KB, Erjefalt J, Bai C. Airway epithelial dysfunction in the development of acute lung injury and acute respiratory distress syndrome. Expert Rev Respir Med. 2007;1:149–55.
Wang Y, Bai C, Li K, Adler KB, Wang X. Role of airway epithelial cells in development of asthma and allergic rhinitis. Respir Med. 2008;102:949–55.
Wang T, Liu Y, Chen L, Wang X, Hu XR, Feng YL, et al. Effect of sildenafil on acrolein-induced airway inflammation and mucus production in rats. Eur Respir J. 2009;33(5):1122–32.
You K, Yang HT, Kym D, Yoon J, Haejun Y, Cho YS, et al. Inhalation injury in burn patients: establishing the link between diagnosis and prognosis. Burns. 2014;40:1470–5.
Funding
This work was supported by the grants from the National Institute of Health (NIH HL134828) and the National Natural Science Foundation of China (NSFC 81670010).
Author information
Authors and Affiliations
Contributions
Hong-Long Ji conceived study, designed experiments, edited manuscript, and approved submission. Jianjun Chang, Zaixing Chen, and Hong-Guang Nie performed experiments, analyzed data, and plotted graphs. Jianjun Chang, Runzhen Zhao, Hong-Guang Nie, and Zaixing Chen prepared manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 3700 kb)
Rights and permissions
About this article
Cite this article
Chang, J., Chen, Z., Zhao, R. et al. Ion transport mechanisms for smoke inhalation–injured airway epithelial barrier. Cell Biol Toxicol 36, 571–589 (2020). https://doi.org/10.1007/s10565-020-09545-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10565-020-09545-1