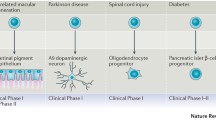
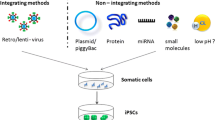
Abstract
Aging, injuries, and diseases can be considered as the result of malfunctioning or damaged cells. Regenerative medicine aims to restore tissue homeostasis by repairing or replacing cells, tissues, or damaged organs, by linking and combining different disciplines including engineering, technology, biology, and medicine. To pursue these goals, the discipline is taking advantage of pluripotent stem cells (PSCs), a peculiar type of cell possessing the ability to differentiate into every cell type of the body. Human PSCs can be isolated from the blastocysts and maintained in culture indefinitely, giving rise to the so-called embryonic stem cells (ESCs). However, since 2006, it is possible to restore in an adult cell a pluripotent ESC-like condition by forcing the expression of four transcription factors with the rejuvenating reprogramming technology invented by Yamanaka. Then the two types of PSC can be differentiated, using standardized protocols, towards the cell type necessary for the regeneration. Although the use of these derivatives for therapeutic transplantation is still in the preliminary phase of safety and efficacy studies, a lot of efforts are presently taking place to discover the biological mechanisms underlying genetic pathologies, by differentiating induced PSCs derived from patients, and new therapies by challenging PSC-derived cells in drug screening.

Similar content being viewed by others
References
Aasen T, Raya A, Barrero MJ, Garreta E, Consiglio A, et al. Efficient and rapid generation of induced pluripotent stem cells from human keratinocytes. Nat Biotechnol. 2008;26(11):1276–84.
Agulnick AD, Ambruzs DM, Moorman MA, Bhoumik A, Cesario RM, et al. Insulin-producing endocrine cells differentiated in vitro from human embryonic stem cells function in macroencapsulation devices in vivo. Stem Cells Transl Med. 2015;4(10):1214–22.
Aiuti A, Biasco L, Scaramuzza S, Ferrua F, Cicalese MP, Baricordi C, et al. Lentiviral hematopoietic stem cell gene therapy in patients with wiskott-aldrich syndrome. Science. 2013;341(6148):1233151-1233151.
Annas GJ, Caplan A, Elias S. The politics of human-embryo research—avoiding ethical gridlock. N Engl J Med. 1996;334(20):1329–32.
Ban H, Nishishita N, Fusaki N, Tabata T, Saeki K, et al. Efficient generation of transgene-free human induced pluripotent stem cells (iPSCs) by temperature-sensitive Sendai virus vectors. Proc Natl Acad Sci U S A. 2011;108(34):14234–9.
Bhonde RR, Sheshadri P, Sharma S, Kumar A. Making surrogate β-cells from mesenchymal stromal cells: perspectives and future endeavors. Int J Biochem Cell Biol. 2014;46:90–102.
Biffi A, Montini E, Lorioli L, Cesani M, Fumagalli F, et al. Lentiviral hematopoietic stem cell gene therapy benefits metachromatic leukodystrophy. Science. 2013;341(6148):1233158.
Bitzer M, Armeanu S, Lauer UM, Neubert WJ. Sendai virus vectors as an emerging negative-strand RNA viral vector system. J Gene Med. 2003;5(7):543–53.
Brouwer M, Zhou H, Nadif KN. Choices for induction of pluripotency: recent developments in human induced pluripotent stem cell reprogramming strategies. Stem Cell Rev. 2016;12(1):54–72.
Bulic-Jakus F, Katusic Bojanac A, Juric-Lekic G, Vlahovic M, Sincic N. Teratoma: from spontaneous tumors to the pluripotency/malignancy assay. Wiley Interdiscip Rev Dev Biol. 2016;5(2):186–209.
Chang CW, Lai YS, Pawlik KM, Liu K, Sun CW, et al. Polycistronic lentiviral vector for “hit and run” reprogramming of adult skin fibroblasts to induced pluripotent stem cells. Stem Cells. 2009;27(5):1042–9.
Choi YS, Dusting GJ, Stubbs S, Arunothayaraj S, Han XL, et al. Differentiation of human adipose-derived stem cells into beating cardiomyocytes. J Cell Mol Med. 2010;14(4):878–89.
Choi SM, Kim Y, Shim JS, Park JT, Wang RH, et al. Efficient drug screening and gene correction for treating liver disease using patient-specific stem cells. Hepatology. 2013;57(6):2458–68.
Chung YG, Eum JH, Lee JE, Shim SH, Sepilian V, et al. Human somatic cell nuclear transfer using adult cells. Cell Stem Cell. 2014;14(6):777–80.
Crotti L, Celano G, Dagradi F, Schwartz PJ. Congenital long QT syndrome. Orphanet J Rare Dis. 2008;3:18.
Davis RL, Weintraub H, Lassar AB. Expression of a single transfected cDNA converts fibroblasts to myoblasts. Cell. 1987;51(6):987–1000.
Dell'Era P, Benzoni P, Crescini E, Valle M, Xia E, et al. Cardiac disease modeling using induced pluripotent stem cell-derived human cardiomyocytes. World J Stem Cells. 2015;7(2):329–42.
Doerflinger RM. The ethics of funding embryonic stem cell research: a Catholic viewpoint. Kennedy Inst Ethics J. 1999;9(2):137–50.
Drawnel FM, Boccardo S, Prummer M, Delobel F, Graff A, et al. Disease modeling and phenotypic drug screening for diabetic cardiomyopathy using human induced pluripotent stem cells. Cell Rep. 2014;9(3):810–21.
Dye BR, Hill DR, Ferguson MA, Tsai YH, Nagy MS, et al. In vitro generation of human pluripotent stem cell derived lung organoids. Elife. 2015;4.
Egawa N, Kitaoka S, Tsukita K, Naitoh M, Takahashi K, et al. Drug screening for ALS using patient-specific induced pluripotent stem cells. Sci Transl Med. 2012;4(145):145ra104.
Evans MJ, Kaufman MH. Establishment in culture of pluripotential cells from mouse embryos. Nature. 1981;292(5819):154–6.
Fusaki N, Ban H, Nishiyama A, Saeki K, Hasegawa M. Efficient induction of transgene-free human pluripotent stem cells using a vector based on Sendai virus, an RNA virus that does not integrate into the host genome. Proc Jpn Acad Ser B Phys Biol Sci. 2009;85(8):348–62.
Giri S, Bader A. A low-cost, high-quality new drug discovery process using patient-derived induced pluripotent stem cells. Drug Discov Today. 2015;20(1):37–49.
González F, Boué S, Izpisúa Belmonte JC. Methods for making induced pluripotent stem cells: reprogramming à la carte. Nat Rev Genet. 2011;12(4):231–42.
Grabundzija I, Wang J, Sebe A, Erdei Z, Kajdi R, et al. Sleeping beauty transposon-based system for cellular reprogramming and targeted gene insertion in induced pluripotent stem cells. Nucleic Acids Res. 2013;41(3):1829–47.
Hockemeyer D, Soldner F, Cook EG, Gao Q, Mitalipova M, Jaenisch R. A drug-inducible system for direct reprogramming of human somatic cells to pluripotency. Cell Stem Cell. 2008;3(3):346-353.
Ilic D, Devito L, Miere C, Codognotto S. Human embryonic and induced pluripotent stem cells in clinical trials. Br Med Bull. 2015;116:19–27.
Jia F, Wilson KD, Sun N, Gupta DM, Huang M, et al. A nonviral minicircle vector for deriving human iPS cells. Nat Methods. 2010;7(3):197–9.
Kim D, Kim CH, Moon JI, Chung YG, Chang MY, et al. Generation of human induced pluripotent stem cells by direct delivery of reprogramming proteins. Cell Stem Cell. 2009;4(6):472–6.
Klimanskaya I, Hipp J, Rezai KA, West M, Atala A, et al. Derivation and comparative assessment of retinal pigment epithelium from human embryonic stem cells using transcriptomics. Cloning Stem Cells. 2004;6(3):217–45.
Ko HC, Gelb BD. Concise review: drug discovery in the age of the induced pluripotent stem cell. Stem Cells Transl Med. 2014;3(4):500–9.
Kulessa H, Frampton J, Graf T. GATA-1 reprograms avian myelomonocytic cell lines into eosinophils, thromboblasts, and erythroblasts. Genes Dev. 1995;9(10):1250–62.
Lancaster MA, Knoblich JA. Organogenesis in a dish: modeling development and disease using organoid technologies. Science. 2014;345(6194):1247125.
Lancaster MA, Renner M, Martin CA, Wenzel D, Bicknell LS, et al. Cerebral organoids model human brain development and microcephaly. Nature. 2013;501(7467):373–9.
Lebkowski J. GRNOPC1: the world’s first embryonic stem cell-derived therapy interview with Jane Lebkowski. Regen Med. 2011;6(6 Suppl):11–3.
Lee MO, Moon SH, Jeong HC, Yi JY, Lee TH, et al. Inhibition of pluripotent stem cell-derived teratoma formation by small molecules. Proc Natl Acad Sci U S A. 2013;110(35):E3281–90.
Lu B, Malcuit C, Wang S, Girman S, Francis P, et al. Long-term safety and function of RPE from human embryonic stem cells in preclinical models of macular degeneration. Stem Cells. 2009;27(9):2126–35.
Maherali N, Hochedlinger K. Guidelines and techniques for the generation of induced pluripotent stem cells. Cell Stem Cell. 2008;3(6):595–605.
Maherali N, Ahfeldt T, Rigamonti A, Utikal J, Cowan C, et al. A high-efficiency system for the generation and study of human induced pluripotent stem cells. Cell Stem Cell. 2008;3(3):340–5.
Mallon BS, Hamilton RS, Kozhich OA, Johnson KR, Fann YC, et al. Comparison of the molecular profiles of human embryonic and induced pluripotent stem cells of isogenic origin. Stem Cell Res. 2014;12(2):376–86.
Martin GR. Isolation of a pluripotent cell line from early mouse embryos cultured in medium conditioned by teratocarcinoma stem cells. Proc Natl Acad Sci U S A. 1981;78(12):7634–8.
Matreyek KA, Engelman A. Viral and cellular requirements for the nuclear entry of retroviral preintegration nucleoprotein complexes. Viruses. 2013;5(10):2483–511.
Mitani K, Kubo S. Adenovirus as an integrating vector. Curr Gene Ther. 2002;2(2):135–44. Review
Mitsui K, Tokuzawa Y, Itoh H, Segawa K, Murakami M, et al. The homeoprotein Nanog is required for maintenance of pluripotency in mouse epiblast and ES cells. Cell. 2003;113(5):631–42.
Moretti A, Bellin M, Welling A, Jung CB, Lam JT, et al. Patient-specific induced pluripotent stem-cell models for long-QT syndrome. N Engl J Med. 2010;363(15):1397–409.
Nakanishi M, Otsu M. Development of Sendai virus vectors and their potential applications in gene therapy and regenerative medicine. Curr Gene Ther. 2012;12(5):410–6.
Nakano T, Ando S, Takata N, Kawada M, Muguruma K, et al. Self-formation of optic cups and storable stratified neural retina from human ESCs. Cell Stem Cell. 2012;10(6):771–85.
Narsinh KH, Jia F, Robbins RC, Kay MA, Longaker MT, et al. Generation of adult human induced pluripotent stem cells using nonviral minicircle DNA vectors. Nat Protoc. 2011;6(1):78–88.
Niwa H. Molecular mechanism to maintain stem cell renewal of ES cells. Cell Struct Funct. 2001;26(3):137–48.
Nutt SL, Heavey B, Rolink AG, Busslinger M. Pillars article: commitment to the B-lymphoid lineage depends on the transcription factor Pax5. Nature. 1999;401: 556–562. J Immunol. 2015;195(3):766–72.
Park HJ, Shin J, Kim J, Cho SW. Nonviral delivery for reprogramming to pluripotency and differentiation. Arch Pharm Res. 2014;37(1):107–19.
Ramalingam S, London V, Kandavelou K, Cebotaru L, Guggino W, et al. Generation and genetic engineering of human induced pluripotent stem cells using designed zinc finger nucleases. Stem Cells Dev. 2013;22(4):595–610.
Robertson JA. Human embryonic stem cell research: ethical and legal issues. Nat Rev Genet. 2001;2(1):74–8. Review
Sánchez-Danés A, Richaud-Patin Y, Carballo-Carbajal I, Jiménez-Delgado S, Caig C, et al. Disease-specific phenotypes in dopamine neurons from human iPS-based models of genetic and sporadic Parkinson’s disease. EMBO Mol Med. 2012;4(5):380–95.
Schwartz SD, Regillo CD, Lam BL, Eliott D, Rosenfeld PJ, et al. Human embryonic stem cell-derived retinal pigment epithelium in patients with age-related macular degeneration and Stargardt's macular dystrophy: follow-up of two open-label phase 1/2 studies. Lancet. 2015;385(9967):509–16.
Scuteri A, Miloso M, Foudah D, Orciani M, Cavaletti G, et al. Mesenchymal stem cells neuronal differentiation ability: a real perspective for nervous system repair? Curr Stem Cell Res Ther. 2011;6(2):82–92.
Solnica-Krezel L, Sepich DS. Gastrulation: making and shaping germ layers. Annu Rev Cell Dev Biol. 2012;28:687–717.
Spence JR, Mayhew CN, Rankin SA, Kuhar MF, Vallance JE, et al. Directed differentiation of human pluripotent stem cells into intestinal tissue in vitro. Nature. 2011;470(7332):105–9.
Stadtfeld M, Hochedlinger K. Induced pluripotency: history, mechanisms, and applications. Genes Dev. 2010;24(20):2239–63.
Stadtfeld M, Nagaya M, Utikal J, Weir G, Hochedlinger K. Induced pluripotent stem cells generated without viral integration. Science. 2008;322(5903):945–9.
Struhl G. A homoeotic mutation transforming leg to antenna in Drosophila. Nature. 1981;292(5824):635–8.
Tachibana M, Amato P, Sparman M, Gutierrez NM, Tippner-Hedges R, et al. Human embryonic stem cells derived by somatic cell nuclear transfer. Cell. 2013;153(6):1228–38.
Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126(4):663–76.
Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131(5):861–72.
Takebe T, Zhang RR, Koike H, Kimura M, Yoshizawa E, et al. Generation of a vascularized and functional human liver from an iPSC-derived organ bud transplant. Nat Protoc. 2014;9(2):396–409.
Tang C, Lee AS, Volkmer JP, Sahoo D, Nag D, et al. An antibody against SSEA-5 glycan on human pluripotent stem cells enables removal of teratoma-forming cells. Nat Biotechnol. 2011;29(9):829–34.
Tapscott SJ, Davis RL, Thayer MJ, Cheng PF, Weintraub H, et al. MyoD1: a nuclear phosphoprotein requiring a Myc homology region to convert fibroblasts to myoblasts. Science. 1988;242(4877):405–11.
Thomson JA, Itskovitz-Eldor J, Shapiro SS, Waknitz MA, Swiergiel JJ, et al. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282(5391):1145–7.
Wobus AM, Löser P. Present state and future perspectives of using pluripotent stem cells in toxicology research. Arch Toxicol. 2011;85(2):79–117.
Woltjen K, Michael IP, Mohseni P, Desai R, Mileikovsky M, et al. piggyBac transposition reprograms fibroblasts to induced pluripotent stem cells. Nature. 2009;458(7239):766–70.
Wu XB, Tao R. Hepatocyte differentiation of mesenchymal stem cells. Hepatobiliary Pancreat Dis Int. 2012;11(4):360–71.
Xie H, Ye M, Feng R, Graf T. Stepwise reprogramming of B cells into macrophages. Cell. 2004;117(5):663–76.
Yakubov E, Rechavi G, Rozenblatt S, Givol D. Reprogramming of human fibroblasts to pluripotent stem cells using mRNA of four transcription factors. Biochem Biophys Res Commun. 2010;394(1):189–93.
Yoshioka N, Gros E, Li H-R, Kumar S, Deacon DC, Maron C, et al. Efficient generation of human iPSCs by a synthetic self-replicative RNA. Cell Stem Cell. 2013;13(2):246-254.
Yu J, Hu K, Smuga-Otto K, Tian S, Stewart R, et al. Human induced pluripotent stem cells free of vector and transgene sequences. Science. 2009;324(5928):797-801.
Yu J, Vodyanik MA, Smuga-Otto K, Antosiewicz-Bourget J, Frane JL, et al. Induced pluripotent stem cell lines derived from human somatic cells. Science. 2007;318(5858):1917–20.
Zhong X, Gutierrez C, Xue T, Hampton C, Vergara MN, et al. Generation of three-dimensional retinal tissue with functional photoreceptors from human iPSCs. Nat Commun. 2014;5:4047.
Zhou W, Freed CR. Adenoviral gene delivery can reprogram human fibroblasts to induced pluripotent stem cells. Stem Cells. 2009;27(11):2667–74.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This work was supported by a grant by Fondazione Cariplo to P.D.E. (ref. no. 2014-0822) and by BFU2013-49157-P and RETICTerCel grants from MINECO and the European Research Council (ERC) 2012-StG (311736- PD-HUMMODEL) to A.C.
Rights and permissions
About this article
Cite this article
Mora, C., Serzanti, M., Consiglio, A. et al. Clinical potentials of human pluripotent stem cells. Cell Biol Toxicol 33, 351–360 (2017). https://doi.org/10.1007/s10565-017-9384-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10565-017-9384-y