Abstract
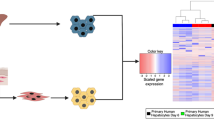
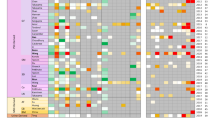
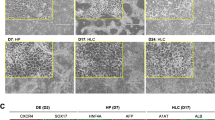
Hepatocytes derived from human induced pluripotent stem cells (iPSCs) hold great promise as an in vitro liver model by virtue of their unlimited long-term supply, stability and consistency in functionality, and affordability of donor diversity. However, the suitability of iPSC-derived hepatocytes (iPSC-Heps) for toxicology studies has not been fully validated. In the current study, we characterized global gene expression profiles of iPSC-Heps in comparison to those of primary human hepatocytes (PHHs) and several human hepatoma cell lines (HepaRG, HuH-7, HepG2, and HepG2/C3A). Furthermore, genes associated with hepatotoxicity, drug-metabolizing enzymes, transporters, and nuclear receptors were extracted for more detailed comparisons. Our results showed that iPSC-Heps correlate more closely to PHHs than hepatoma cell lines, suggesting that iPSC-Heps had a relatively mature hepatic phenotype that more closely resembles that of adult hepatocytes. HepaRG was the sole exception but nonetheless suffers from lack of donor diversity and poor prediction of hepatotoxicity. The effects of sex differences and DMSO treatment on gene expression of the cellular models were also investigated. Overall, the results presented in the current study suggest that iPSC-Heps represent a reproducible source of human hepatocytes and a promising in vitro model for hepatotoxicity evaluation. Further studies are needed to develop a robust protocol for hepatocyte differentiation towards a more mature adult phenotype.






Similar content being viewed by others
Abbreviations
- ANOVA:
-
Analysis of variance
- cDNA:
-
Complimentary deoxyribonucleic acid
- cRNA:
-
Complimentary ribonucleic acid
- CYP:
-
Cytochrome P450
- DEG:
-
Differentially expressed gene
- DMEM:
-
Dulbecco’s Modified Eagle Medium
- DMSO:
-
Dimethyl sulfoxide
- FC:
-
Fold change
- FDR:
-
False discovery rate
- HCA:
-
Hierarchical clustering analysis
- HEPES :
-
4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid
- iPSC:
-
Induced pluripotent stem cell
- iPSC-Hep:
-
Induced pluripotent stem cell-derived hepatocyte
- IVT:
-
In vitro transcription
- MEM:
-
Minimal essential medium
- PCA:
-
Principal component analysis
- PHH:
-
Primary human hepatocyte
- RIN:
-
RNA integrity number
- RMA:
-
Robust multi-array average (algorithm)
- RPMI:
-
Roswell Park Memorial Institute (medium)
References
Aden DP, Fogel A, Plotkin S, Damjanov I, Knowles BB. Controlled synthesis of HBsAg in a differentiated human liver carcinoma-derived cell line. Nature. 1979;282:615–6.
Andersson TB, Kanebratt KP, Kenna JG. The HepaRG cell line: a unique in vitro tool for understanding drug metabolism and toxicology in human. Expert Opin Drug Metab Toxicol. 2012;8:909–20.
Aninat C, Piton A, Glaise D, Le Charpentier T, Langouet S, Morel F, Guguen-Guillouzo C, Guillouzo A. Expression of cytochromes P450, conjugating enzymes and nuclear receptors in human hepatoma HepaRG cells. Drug metabolism and disposition: the biological fate of chemicals. 2006;34:75–83.
Asgari S, Pournasr B, Salekdeh GH, Ghodsizadeh A, Ott M, Baharvand H. Induced pluripotent stem cells: a new era for hepatology. J Hepatol. 2010;53:738–51.
Berger DR, Ware BR, Davidson MD, Allsup SR, Khetani SR. Enhancing the functional maturity of induced pluripotent stem cell-derived human hepatocytes by controlled presentation of cell-cell interactions in vitro. Hepatology (Baltimore, Md). 2015;61:1370–81.
Cheng X, Buckley D, Klaassen CD. Regulation of hepatic bile acid transporters Ntcp and Bsep expression. Biochem Pharmacol. 2007;74:1665–76.
Choi S, Sainz Jr B, Corcoran P, Uprichard S, Jeong H. Characterization of increased drug metabolism activity in dimethyl sulfoxide (DMSO)-treated Huh7 hepatoma cells. Xenobiotica. 2009;39:205–17.
Cipriano, M., Correia, J.C., Camoes, S.P., Oliveira, N.G., Cruz, P., Cruz, H., Castro, M., Ruas, J.L., Santos, J.M., and Miranda, J.P. (2016). The role of epigenetic modifiers in extended cultures of functional hepatocyte-like cells derived from human neonatal mesenchymal stem cells. Archives of toxicology.
Czysz K, Minger S, Thomas N. DMSO efficiently down regulates pluripotency genes in human embryonic stem cells during definitive endoderm derivation and increases the proficiency of hepatic differentiation. PLoS One. 2015;10:e0117689.
Darling D, Tavassoli M, Linskens MH, Farzaneh F. DMSO induced modulation of c-myc steady-state RNA levels in a variety of different cell lines. Oncogene. 1989;4:175–9.
Flynn TJ, Ferguson MS. Multiendpoint mechanistic profiling of hepatotoxicants in HepG2/C3A human hepatoma cells and novel statistical approaches for development of a prediction model for acute hepatotoxicity. Toxicol in Vitro. 2008;22:1618–31.
Gerets HH, Tilmant K, Gerin B, Chanteux H, Depelchin BO, Dhalluin S, Atienzar FA. Characterization of primary human hepatocytes, HepG2 cells, and HepaRG cells at the mRNA level and CYP activity in response to inducers and their predictivity for the detection of human hepatotoxins. Cell Biol Toxicol. 2012;28:69–87.
Gieseck 3rd RL, Hannan NR, Bort R, Hanley NA, Drake RA, Cameron GW, Wynn TA, Vallier L. Maturation of induced pluripotent stem cell derived hepatocytes by 3D-culture. PLoS One. 2014;9:e86372.
Gripon P, Rumin S, Urban S, Le Seyec J, Glaise D, Cannie I, Guyomard C, Lucas J, Trepo C, Guguen-Guillouzo C. Infection of a human hepatoma cell line by hepatitis B virus. Proc Natl Acad Sci U S A. 2002;99:15655–60.
Guillouzo A, Corlu A, Aninat C, Glaise D, Morel F, Guguen-Guillouzo C. The human hepatoma HepaRG cells: a highly differentiated model for studies of liver metabolism and toxicity of xenobiotics. Chem Biol Interact. 2007;168:66–73.
Hart SN, Li Y, Nakamoto K, Subileau EA, Steen D, Zhong XB. A comparison of whole genome gene expression profiles of HepaRG cells and HepG2 cells to primary human hepatocytes and human liver tissues. Drug metabolism and disposition: the biological fate of chemicals. 2010;38:988–94.
Hewitt NJ, Lechon MJ, Houston JB, Hallifax D, Brown HS, Maurel P, Kenna JG, Gustavsson L, Lohmann C, Skonberg C, et al. Primary hepatocytes: current understanding of the regulation of metabolic enzymes and transporter proteins, and pharmaceutical practice for the use of hepatocytes in metabolism, enzyme induction, transporter, clearance, and hepatotoxicity studies. Drug Metab Rev. 2007;39:159–234.
Holmgren G, Sjogren AK, Barragan I, Sabirsh A, Sartipy P, Synnergren J, Bjorquist P, Ingelman-Sundberg M, Andersson TB, Edsbagge J. Long-term chronic toxicity testing using human pluripotent stem cell-derived hepatocytes. Drug metabolism and disposition: the biological fate of chemicals. 2014;42:1401–6.
Irizarry RA, Hobbs B, Collin F, Beazer-Barclay YD, Antonellis KJ, Scherf U, Speed TP. Exploration, normalization, and summaries of high density oligonucleotide array probe level data. Biostatistics (Oxford, England). 2003;4:249–64.
Isom HC, Secott T, Georgoff I, Woodworth C, Mummaw J. Maintenance of differentiated rat hepatocytes in primary culture. Proc Natl Acad Sci U S A. 1985;82:3252–6.
Jacob SW, Herschler R. Pharmacology of DMSO. Cryobiology. 1986;23:14–27.
Kennedy RH. In vitro toxicology testing: it’s time to report the sex of cells. Toxicol Forensic Med Open J. 2016;1:e5–8.
Koturbash I, Scherhag A, Sorrentino J, Sexton K, Bodnar W, Swenberg JA, Beland FA, Pardo-Manuel Devillena F, Rusyn I, Pogribny IP. Epigenetic mechanisms of mouse interstrain variability in genotoxicity of the environmental toxicant 1,3-butadiene. Toxicol Sci. 2011;122:448–56.
Liu J, Yoshikawa H, Nakajima Y, Tasaka K. Involvement of mitochondrial permeability transition and caspase-9 activation in dimethyl sulfoxide-induced apoptosis of EL-4 lymphoma cells. Int Immunopharmacol. 2001;1:63–74.
Liu Y, Flynn TJ, Xia M, Wiesenfeld PL, Ferguson MS. Evaluation of CYP3A4 inhibition and hepatotoxicity using DMSO-treated human hepatoma HuH-7 cells. Cell Biol Toxicol. 2015;31:221–30.
Lu J, Einhorn S, Venkatarangan L, Miller M, Mann DA, Watkins PB, LeCluyse E. Morphological and functional characterization and assessment of iPSC-derived hepatocytes for in vitro toxicity testing. Toxicol Sci. 2015;147:39–54.
Maliepaard M, Scheffer GL, Faneyte IF, van Gastelen MA, Pijnenborg AC, Schinkel AH, van De Vijver MJ, Scheper RJ, Schellens JH. Subcellular localization and distribution of the breast cancer resistance protein transporter in normal human tissues. Cancer Res. 2001;61:3458–64.
Mann DA. Human induced pluripotent stem cell-derived hepatocytes for toxicology testing. Expert Opin Drug Metab Toxicol. 2015;11:1–5.
Medine CN, Lucendo-Villarin B, Storck C, Wang F, Szkolnicka D, Khan F, Pernagallo S, Black JR, Marriage HM, Ross JA, et al. Developing high-fidelity hepatotoxicity models from pluripotent stem cells. Stem cells translational medicine. 2013;2:505–9.
Nagamoto Y, Tashiro K, Takayama K, Ohashi K, Kawabata K, Sakurai F, Tachibana M, Hayakawa T, Furue MK, Mizuguchi H. The promotion of hepatic maturation of human pluripotent stem cells in 3D co-culture using type I collagen and Swiss 3 T3 cell sheets. Biomaterials. 2012;33:4526–34.
Nakabayashi H, Taketa K, Miyano K, Yamane T, Sato J. Growth of human hepatoma cells lines with differentiated functions in chemically defined medium. Cancer Res. 1982;42:3858–63.
Raab S, Klingenstein M, Liebau S, Linta L. A comparative view on human somatic cell sources for iPSC generation. Stem Cells Int. 2014;2014:768391.
Richert L, Liguori MJ, Abadie C, Heyd B, Mantion G, Halkic N, Waring JF. Gene expression in human hepatocytes in suspension after isolation is similar to the liver of origin, is not affected by hepatocyte cold storage and cryopreservation, but is strongly changed after hepatocyte plating. Drug metabolism and disposition: the biological fate of chemicals. 2006;34:870–9.
Ringner M. What is principal component analysis? Nat Biotechnol. 2008;26:303–4.
Sakai Y, Jiang J, Kojima N, Kinoshita T, Miyajima A. Enhanced in vitro maturation of fetal mouse liver cells with oncostatin M, nicotinamide, and dimethyl sulfoxide. Cell Transplant. 2002;11:435–41.
Santos NC, Figueira-Coelho J, Martins-Silva J, Saldanha C. Multidisciplinary utilization of dimethyl sulfoxide: pharmacological, cellular, and molecular aspects. Biochem Pharmacol. 2003;65:1035–41.
Sassa S, Sugita O, Galbraith RA, Kappas A. Drug metabolism by the human hepatoma cell, Hep G2. Biochem Biophys Res Commun. 1987;143:52–7.
Schwartz RE, Fleming HE, Khetani SR, Bhatia SN. Pluripotent stem cell-derived hepatocyte-like cells. Biotechnol Adv. 2014;32:504–13.
Si-Tayeb, K., Noto, F.K., Nagaoka, M., Li, J., Battle, M.A., Duris, C., North, P.E., Dalton, S., and Duncan, S.A. (2010). Highly efficient generation of human hepatocyte-like cells from induced pluripotent stem cells. Hepatology (Baltimore, Md.) 51, 297–305.
Singh VK, Kumar N, Kalsan M, Saini A, Chandra R. Mechanism of induction: induced pluripotent stem cells (iPSCs). Journal of stem cells. 2015;10:43–62.
Sirenko O, Hesley J, Rusyn I, Cromwell EF. High-content assays for hepatotoxicity using induced pluripotent stem cell-derived cells. Assay Drug Dev Technol. 2014;12:43–54.
Srinivas S, Sironmani TA, Shanmugam G. Dimethyl sulfoxide inhibits the expression of early growth-response genes and arrests fibroblasts at quiescence. Exp Cell Res. 1991;196:279–86.
Sullivan, G.J., Hay, D.C., Park, I.H., Fletcher, J., Hannoun, Z., Payne, C.M., Dalgetty, D., Black, J.R., Ross, J.A., Samuel, K., et al. (2010). Generation of functional human hepatic endoderm from human induced pluripotent stem cells. Hepatology (Baltimore, Md.) 51, 329–335.
Sun C, Wilson GS, Fan JG, Qiao L. Potential applications of induced pluripotent stem cells (iPSCs) in hepatology research. Current Stem Cell Research & Therapy. 2015;10:208–15.
Suter-Dick L, Alves PM, Blaauboer BJ, Bremm KD, Brito C, Coecke S, Flick B, Fowler P, Hescheler J, Ingelman-Sundberg M, et al. Stem cell-derived systems in toxicology assessment. Stem Cells Dev. 2015;24:1284–96.
Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–76.
Takayama K, Kawabata K, Nagamoto Y, Kishimoto K, Tashiro K, Sakurai F, Tachibana M, Kanda K, Hayakawa T, Furue MK, et al. 3D spheroid culture of hESC/hiPSC-derived hepatocyte-like cells for drug toxicity testing. Biomaterials. 2013;34:1781–9.
Tong W, Cao X, Harris S, Sun H, Fang H, Fuscoe J, Harris A, Hong H, Xie Q, Perkins R, et al. ArrayTrack—supporting toxicogenomic research at the U.S. Food and Drug Administration National Center for toxicological research. Environ Health Perspect. 2003;111:1819–26.
Wang B, Jakus AE, Baptista PM, Soker S, Soto-Gutierrez A, Abecassis MM, Shah RN, Wertheim JA. Functional maturation of induced pluripotent stem cell hepatocytes in extracellular matrix-a comparative analysis of bioartificial liver microenvironments. Stem cells translational medicine. 2016;5:1257–67.
Ward Jr JH. Hierarchical grouping to optimize an objective function. J Am Stat Assoc. 1963;58:236–44.
Ware BR, Berger DR, Khetani SR. Prediction of drug-induced liver injury in Micropatterned Co-cultures containing iPSC-derived human hepatocytes. Toxicol Sci. 2015;145:252–62.
Xu JJ, Diaz D, O'Brien PJ. Applications of cytotoxicity assays and pre-lethal mechanistic assays for assessment of human hepatotoxicity potential. Chem Biol Interact. 2004;150:115–28.
Acknowledgement
The authors thank Drs. Thomas J. Flynn and Menghang Xia for providing primary human hepatocytes and HepG2 cells, respectively, and Dr. Michael F. Santillo for critical review of the manuscript. The authors also thank Drs. Jeffrey J. Yourick, Paddy L. Wiesenfeld, and Robert L. Sprando for their support and guidance on this work. The findings and conclusions presented in this article are those of the authors and do not necessarily represent views, opinions, or policies of the U.S. Food and Drug Administration.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest statement
The authors declare that they have no conflict of interest.
Additional information
Xiugong Gao and Yitong Liu contributed equally to the study.
Electronic supplementary material
Supplementary Table 1
(XLSX 9 kb)
Supplementary Table 2
(XLSX 24 kb)
Supplementary Table 3
(XLSX 22 kb)
Supplementary Table 4
(XLSX 24 kb)
Rights and permissions
About this article
Cite this article
Gao, X., Liu, Y. A transcriptomic study suggesting human iPSC-derived hepatocytes potentially offer a better in vitro model of hepatotoxicity than most hepatoma cell lines. Cell Biol Toxicol 33, 407–421 (2017). https://doi.org/10.1007/s10565-017-9383-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10565-017-9383-z