Abstract
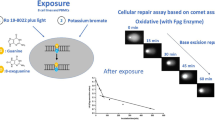
It has been suggested that extended-term cultures of human lymphocytes could be used as a complement to cell lines based on transformed cells when testing the genotoxicity of chemicals. To investigate whether the pattern of induced DNA damage and its subsequent repair differs significantly between cultures based on different blood donors, hydrogen peroxide (H2O2)-induced DNA damage was measured in cultures from four different subjects using the comet assay. The DNA damage was significantly increased in all cultures after 10 min exposure to 0.25 mmol/L H2O2, and there was a significant decrease in the H2O2-induced DNA damage in all cultures after 30 min of DNA repair. The level of damage varied between the different donors, especially after the repair. Using PCR and DNA sequencing, exon 5 of the p53 gene was sequenced in the lymphocytes from the donors with the lowest and highest residual damage. No such mutation was found. Mouse lymphoma L5178Y cells carrying the p53 mutation in exon 5 were included as a reference. These cells were found to be less sensitive toward the H2O2-induced DNA damage, and they were also found to have a rather low DNA repair capacity. The demonstrated variation in H2O2-induced DNA damage and DNA repair capacity between the cultures from the different subjects may be important from a risk assessment perspective, but is obviously not of decisive importance when it comes to the development of a routine assay for genotoxicity.
Similar content being viewed by others
Abbreviations
- HIFCS:
-
heat-inactivated fetal calf serum
- PCR:
-
polymerase chain reaction
- PHA:
-
phytohemagglutinin
References
Achanta G, Huang P. Role of p53 in sensing oxidative DNA damage in response to reactive oxygen species-generating agents. Cancer Res 2004;64:6233–9.
Andersson M, Hellman B. Different roles of Fpg and Endo III on catechol-induced DNA damage in extended-term cultures of human lymphocytes and L5178Y mouse lymphoma cells. Toxicology In Vitro 2005;19:779–86.
Andersson M, Agurell E, Vaghef H, Bolcsfoldi G, Hellman B. Extended-term cultures of human T-lymphocytes and the comet assay: a useful combination when testing for genotoxicity in vitro? Mutat Res 2003;540:43–55.
Andreassen PR, Ho G, D’Andrea AD. DNA damage responses and their many interactions with the replication fork. Carcinogenesis 2006;27:883–92.
Bergqvist M, Brattström D, Stålberg M, Vaghef H, Brodin O, Hellman B. Evaluation of radiation-induced DNA damage and DNA repair in human lung cancer cell lines with different radiosensitivity using alkaline and neutral single cell gel electrophoresis. Cancer Lett 1998;133:9–18.
Berwick M, Vineis P. Markers of DNA repair and susceptibility to cancer in humans: an epidemiologic review. J Natl Cancer Inst 2000;92:874–97.
Bock C, Dittmar H, Gemeinhardt H, Bauer E, Greulich K-O. Comet assay detects cold repair of UV-A damages in a human B-lymphoblast cell line. Mutat Res 1988;408:111–20.
Clark LS, Hart DW, Vojta PJ, et al. Identification and chromosomal assignment of two heterozygous mutations in the Trp gene in L5178Y/TK+/−-3.7.2C mouse lymphoma cells. Mutagenesis 1998;13:427–34.
Collins A, Harrington V. Repair of oxidative DNA damage: assessing its contribution to cancer prevention. Mutagenesis 2002;17:489–92.
Collins AR. The Comet assay for DNA damage and repair. Mol Biotechnol 2004;26:249–61.
Collins AR, Dusinska M, Horvathova E, Munro E, Savio M, Stetina R. Interindividual differences in repair of DNA base oxidation, measured in vitro with the comet assay. Mutagenesis 2001;16:297–301.
Duthie SJ, Collins AR. The influence of cell growth, detoxifying enzymes and DNA repair on hydrogen peroxide-mediated DNA damage (measured using the Comet assay) in human cells. Free Radic Biol Med 1997;22:717–24.
Frankenberg-Schwager M. Review of repair kinetics for DNA damage induced in eukaryotic cells in vitro by ionizing radiation. Radiother Oncol 1989;14:307–20.
Friedberg E, Walker GC, Siede W. DNA repair and mutagenesis. Washington DC: ASM Press; 1995.
Geske FJ, Nelson, AC, Lieberman R, Strange R, Sun T, Gerschenson LE. DNA repair is activated in early stages of p53-induced apoptosis. Cell Death Differ 2000;7:393–401.
Hartmann A, Speit G. The contribution of cytotoxicity to DNA-effects in the single cell cell gel test (comet assay). Toxicol Lett 1997;90:183–8.
Hellman B, Vaghef H, Boström B. The concepts of tail moment and tail inertia in the single cell gel electrophoresis assay. Mutat Res 1995;336:123–31.
Hellman B, Brodin D, Andersson M, et al. Radiation-induced DNA-damage and gene expression profiles in human lung cancer cell lines with different radiosensitivity. Exp Oncol 2005;27:102–7.
Hemminki K, Xu G, Angelini S, et al. XPD exon 10 and 23 polymorphisms and DNA repair in human skin in situ. Carcinogenesis 2001;22:1185–8.
Hess J, Clark LS, Moore MM. Trp53 sequence analysis of L5178Y cell line derivatives. Environ Mol Mutagen 2003;42:122–4.
Holz O, Jörres R, Kästner A, Krause T, Magnussen H. Reproducibility of basal and induced DNA single-strand breaks detected by the single-cell gel electrophoresis assay in human peripheral mononuclear leukocytes. Int Arch Occup Environ Health 1995;67:305–10.
Hu JJ, Dubin N, Kurland D, Ma BL, Roush GC. The effects of hydrogen peroxide on DNA repair activities. Mutat Res 1995;336:193–201.
Hu JJ, Smith TR, Miller MS, Mohrenweiser HW, Golden A, Case LD. Amino acid substitution variants of APE1 and XRCC1 genes associated with ionizing radiation sensitivity. Carcinogenesis 2001;22:917–22.
Janssen K, Eichhorn-Grombacher U, Schlink K, Nitzsche S, Oesch F, Kaina B. Long-time expression of DNA repair enzymes MGMT and APE in human peripheral blood mononuclear cells. Arch Toxicol 2001;75:306–12.
Kastan MB, Bartek J. Cell-cycle checkpoints and cancer. Nature 2004;432:316–23.
Ljungman M. Individual variation in p53 responsiveness. J Natl Cancer Inst 2001;93:82–3.
Marcon F, Andreoli C, Rossi S, Verdina A, Galati R, Crebelli R. Assessment of individual sensitivity to ionizing radiation and DNA repair efficiency in a healthy population. Mutat Res 1993;541:1–8.
Mayer C, Popanda O, Zelezny O, von Brevern M-C, Bach A, Bartsch H, et al. DNA repair capacity after gamma-irradiation and expression profiles of DNA repair genes in resting and proliferating human peripheral blood lymphocytes. DNA Repair 2002;1:237–50.
McKelvey-Martin VJ, Green MHL, Schmezer P, Pool-Zobel BL, De Méo MP, Collins A. The single cell gel electrophoresis assay (comet assay): a European review. Mutat Res 1993;288:47–63.
O’Donovan MR, Freemantle MR, Hull G, Bell DA, Arlett CF, Cole J. Extended-term cultures of human lymphocytes: a practical alternative to primary human lymphocytes for use in genotoxicity testing. Mutagenesis 1995;10:189–201.
Pero RW, Bryngelsson C, Mitelman F, Kornfält R, Thulin T, Norden Å. Interindividual variation in the responses of cultured human lymphocytes to exposure from DNA damaging agents. Mutat Res 1978;53:327–41.
Petermann E, Keil C, Oei SL. Importance of poly(ADP-ribose) polymerase in the regulation of DNA-dependent processes. Cell Mol Life Sci 2005;62:731–8.
Schmezer P, Rajaee-Behbahani N, Risch A, et al. Rapid screening assay for mutagen sensitivity and DNA repair capacity in human peripheral blood lymphocytes. Mutagenesis 2001;16:25–30.
Setlow RB. Variations in DNA repair among humans. In: Human carcinogenesis. New York: Academic Press; 1983. p. 231–54.
Shechter D, Costanzo V, Gautier J. ATR and ATM regulate the timing of DNA replication origin firing. Nat Cell Biol 2004;6:648–55.
Singh NP, McCoy MT, Tice RR, Schneider EL. A simple technique for quantification of low levels of DNA damage in individual cells. Exp Cell Res 1988;175:184–91.
Smith ML, Seo YR. p43 regulation of DNA excision repair pathways. Mutagenesis 2002;17:149–56.
Storer RD, Kraynak AR, McKelvey TW, Elia MC, Goodrow TL, DeLuca JG. The mouse lymphoma L5178Y Tk+/− cell line is heterozygous for a codon 170 mutation in the p53 tumor suppressor gene. Mutat Res 1997;373:157–65.
Termini J. Hydroperoxide-induced DNA damage and mutations. Mutat Res 2000;450:107–24.
Tice RR, Agurell E, Anderson D, et al. Single cell gel/comet assay: guidelines for in vitro and in vivo genetic toxicology testing. Environ Mol Mutagen 2000;35:206–21.
Torbergsen AC, Collins AR. Recovery of human lymphocytes from oxidative DNA damage: the apparent enhancement of DNA repair is probably simply an antioxidant effect. Eur J Nutr 2000;39:80–5.
Vaghef H, Wisén A-C, Hellman B. Demonstration of benzo(a)pyrene-induced DNA damage in mice by alkaline single cell gel electrophoresis: evidence for strand breaks in liver but not in lymphocytes and bone marrow. Pharmacol Toxicol 1996;78:37–43.
Zhou J, Ahn J, Wilson SH, Prives C. A role for p53 in base excision repair. EMBO J 2001;20:914–23.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Andersson, M., Stenqvist, P. & Hellman, B. Interindividual differences in initial DNA repair capacity when evaluating H2O2-induced DNA damage in extended-term cultures of human lymphocytes using the comet assay. Cell Biol Toxicol 23, 401–411 (2007). https://doi.org/10.1007/s10565-007-9002-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10565-007-9002-5