Abstract
In this study, LiFePO4-carbon (LFP-C) and LFP-C/reduced graphene oxide (rGO) nanocomposites were prepared by ultrasonic spray pyrolysis technique in different calcination conditions to be used as the cathode-active materials for lithium ion battery (LIB). The structure, morphology and composition of the obtained materials were analyzed by X-ray diffraction (XRD), scanning electron microscope (SEM), high-resolution transmission electron microscopy (HR-TEM) and energy-dispersive X-ray spectroscopy (EDX). The XRD results reveal that the olivine pure phase was obtained after calcination of the LFP-C. The SEM images of the prepared materials exhibit the spherical morphology with nanometer size and also change in the morphology by applying the calcination step. The electrochemical performances of cathode-active materials were investigated by charge–discharge test, electrochemical impedance spectroscopy and cyclic voltammetry. The obtained results for LFP-C show that the electrochemical performance was improved by adding carbon precursor and calcining step; in the optimum calcination conditions; 700 °C for 3 h, the LFP-C shows good results in terms of electrochemical performance in comparison with LFP alone. The LFP-C/rGO nanocomposite exhibits the best electrochemical performance however: highest rechargeable capacity and cycle stability; discharge capacity (168 mAh/g at 0.1 C and 123.5 mAh/g at 10 C) and capacity retention of 100% after 50 cycles with maximum reversibility and lithium ion (Li+) diffusion coefficient.
Graphical Abstract
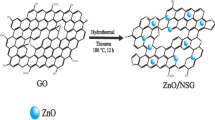
Schematic representation of preparation of the cathode-active materials.











Similar content being viewed by others
References
Armand M, Tarascon J-M (2008) Nature 451:652
Wong Y, Chan C (2012) Vehicle energy storage: batteries. In: Meyers RA (ed) Encyclopedia of sustainability science and technology, Springer, New York, pp. 11502–11522
Yang Z, Zhang J, Kintner-Meyer MC et al (2011) Chem Rev 111:3577
Ravet N, Chouinard Y, Magnan J et al (2001) J Power Sources 97:503
Yang K, Deng Z, Suo J (2012) J Solid State Electrochem 16:2805
Huang H, Yin S-C, Nazar LS (2001) Electrochem Solid-State Lett 4:A170
Croce F, d’Epifanio A, Hassoun J et al (2002) Electrochem Solid-State Lett 5:A47
Chung S-Y, Bloking JT, Chiang Y-M (2002) Nat Mater 1:123
Luo S, Tang Z, Lu JZ et al (2008) Ceram Int 34:1349
Konarova M, Taniguchi I (2010) J Power Sources 195:3661
Kwon SJ, Kim CW, Jeong WT et al (2004) J Power Sources 137:93
Li Y, Wan C, Wu Y et al (2000) J Power Sources 85:294
Park S-H, Oh SW, Sun Y-K (2005) J Power Sources 146:622
Park S-H, Oh S-W, Myung S-T et al (2005) Solid State Ion 176:481
Kang H-C, Jun D-K, Jin B et al (2008) J Power Sources 179:340
Choi D, Kumta PN (2007) J Power Sources 163:1064
Lee S-B, Cho S, Cho S et al (2008) Electrochem Commun 10:1219
Jin EM, Jin B, Jun D-K et al (2008) J Power Sources 178:801
Kim D, Im J, Kang J et al (2007) J Nanosci Nanotechnol 7:3949
Arnold G, Garche J, Hemmer R et al (2003) J Power Sources 119:247
Geim AK, Novoselov KS (2007) Nat Mater 6:183
Zhang LL, Zhou R, Zhao X (2010) J Mater Chem 20:5983
Su C, Bu X, Xu L et al (2012) Electrochim Acta 64:190
Wang L, Wang H, Liu Z et al (2010) Solid State Ion 181:1685
Zhou X, Wang F, Zhu Y et al (2011) J Mater Chem 21:3353
Lu L-M, Qiu X-L, Zhang X-B et al (2013) Biosens Bioelectron 45:102
Guo Y, Guo S, Ren J et al (2010) Acs Nano 4:4001
Kovtyukhova NI, Ollivier PJ, Martin BR et al (1999) Chem Mater 11:771
Kodera T, Bi DY, Ogawa D et al (2011) Key Eng Mater 485:107–110
Yang M-R, Teng T-H, Wu S-H (2006) J Power Sources 159:307
Wang X, Cheng K, Zhang J et al (2013) Adv Powder Tech 24:593
Akao S, Yamada M, Kodera T et al (2010) Int J Chem Eng. https://doi.org/10.1155/2010/175914
Gabrisch H, Wilcox JD, Doeff MM (2006) Electrochem Solid-State Lett 9:A360
Bang J, Didenko Y, Helmich R et al (2012) Aldrich Mater Matter 7:15
Wang J, Sun X (2012) Energ Environ Sci 5:5163
Zhang Y, Feng H, Wu X et al (2009) Electrochim Acta 54:3206
Oh SW, Myung ST, Oh SM et al (2010) Adv Mater 22:4842
Yang J, Wang J, Wang D et al (2012) J Power Sources 208:340
Kim J-K, Choi J-W, Chauhan GS et al (2008) Electrochim Acta 53:8258
Konarova M, Taniguchi I (2008) Mater Res Bull 43:3305
Liao X-Z, Ma Z-F, He Y-S et al (2005) J Electrochem Soc 152:A1969
Wang Y, Wang J, Yang J et al (2006) Adv Func Mater 16:2135
Hanai K, Maruyama T, Imanishi N et al (2008) J Power Sources 178:789
Wang Y, Feng Z-S, Chen J-J et al (2012) Mater Lett 71:54
Scrosati B, Abraham K, Schalkwijk WA et al (2013) Lithium batteries: advanced technologies and applications. Wiley, Hoboken
Kang F-Y, Ma J, Li B-H (2011) New Carbon Mater 26:161
Morgan D, Van der Ven A, Ceder G (2004) Electrochem Solid-State Lett 7:A30
Heinze J (1984) Angew Chem Int Ed 23:831
Jin B, Jin EM, Park K-H et al (2008) Electrochem Commun 10:1537
Liu J, Manthiram A (2009) Chem Mater 21:1695
Huang Y-H, Goodenough JB (2008) Chem Mater 20:7237
Dominko R, Bele M, Gaberscek M et al (2005) J Electrochem Soc 152:A607
Kang B, Ceder G (2009) Nature 458:190
Murugan AV, Muraliganth T, Manthiram A (2008) Electrochem Commun 10:903
Liu J, Kunz M, Chen K et al (2010) J Phys Chem Lett 1:2120
Liu J, Conry TE, Song X et al (2011) Energy Environ Sci 4:885
Talebi-Esfandarani M (2013) Synthesis, Characterization and Modification of LifeP04 by Doping with Platinum and Palladium for Lithium-Ion Batteries (Thèse de doctorat, École Polytechnique de Montréal)
Yu S, Dan S, Luo G et al (2012) J Solid State Electrochem 16:1675
Molenda J, Ojczyk W, Marzec J (2007) J Power Sources 174:689
Acknowledgements
The authors would like to appreciate the University of Azarbaijan Shahid Madani University for providing facilities and financial support.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Mollazadeh, M., Habibi, B. LiFePO4/Carbon/Reduced Graphene Oxide Nanostructured Composite as a High Capacity and Fast Rate Cathode Material for Rechargeable Lithium Ion Battery. Catal Lett 149, 7–18 (2019). https://doi.org/10.1007/s10562-018-2589-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10562-018-2589-8