Abstract
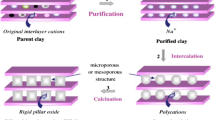
Natural bentonite clay was pillared with Al and Cr polycations (Al-PILCs and Cr-PILCs) as well as with both Al and Cr mixed polycations (Al/Cr-PILCs) at different Al/Cr molar ratios. The structural and textural properties of the pillared clay catalysts were characterized by various techniques, such as high resolution scanning electron microscopy with energy dispersive system, powder X-ray diffraction, adsorption–desorption of N2 at − 196 °C, Fourier transform infrared spectroscopy, thermogravimetric analysis, cation exchange capacity and zeta potential. The obtained results showed that the intercalation of polycations in the interlamellar space of clay led to changes in the clay properties, basal spacing and specific surface area of a mixed PILCs were higher compared to single PILCs. Moreover, diffractograms of the Cr-PILCs confirmed the presence of a Cr2O3 phase. The catalytic wet air oxidation (CWAO) of the phenol was studied in a batchwise slurry reactor, at the experimental conditions of 100 °C temperature, 10 bar oxygen (O2) partial pressure, at pH 3.0, 100 mg/L concentration and 0.4 g of catalyst. All catalysts were analysed to determine the conversion of phenol and removal of total organic carbon (TOC). The results showed that, Al-PILCs displayed higher removal of phenol and TOC conversion than Cr-PILCs. In the case of mixed Al/Cr-PILCs, maximum removal values of phenol and TOC conversion were achieved with Al/Cr molar ratio of 1:1. Moreover, aromatic intermediate products as catechol, hydroquinone and benzoquinones which are identified in the beginning of the phenol oxidation oxidized into short-chain carboxylic acids such as oxalic acid, maleic acid, formic acid, acetic acid and malonic acid. The obtained results demonstrate the potential of Al/Cr-PILCs catalyst as robust and cheap catalyst for CWAO of phenol under mild reaction conditions.
Graphical Abstract









Similar content being viewed by others

References
Ye W, Zhao B, Gao H, Huang J, Zhang X (2016) Preparation of highly efficient and stable Fe,Zn,Al-pillared montmorillonite as heterogeneous catalyst for catalytic wet peroxide oxidation of Orange II. J Porous Mater 23:301–310. https://doi.org/10.1007/s10934-015-0082-y
Sassi H, Lafaye G, Ben Amor H, Gannouni A, Jeday MR, Barbier J (2017) Wastewater treatment by catalytic wet air oxidation process over Al-Fe pillared clays synthesized using microwave irradiation. Front Environ Sci Eng 12:2. https://doi.org/10.1007/s11783-017-0971-1
Zhu J, Wen K, Wang Y, Ma L, Su X, Zhu R, Xi Y, He H (2018) Superior thermal stability of Keggin-Al30pillared montmorillonite: A comparative study with Keggin-Al13pillared montmorillonite. Microporous Mesoporous Mater 265:104–111. https://doi.org/10.1016/j.micromeso.2018.02.007
Li J, Hu M, Zuo S, Wang X (2018) Catalytic combustion of volatile organic compounds on pillared interlayered clay (PILC)-based catalysts. Curr Opin Chem Eng 20:93–98. https://doi.org/10.1016/j.coche.2018.02.001
Moma J, Baloyi J, Ntho T (2018) Synthesis and characterization of an efficient and stable Al/Fe pillared clay catalyst for the catalytic wet air oxidation of phenol. RSC Adv 8:30115–30124. https://doi.org/10.1039/C8RA05825C
Vicente MA, Gil A, Bergaya F (2013) Pillared clays and clay minerals. In: Bergaya F, Lagaly G (eds.) Handbook of Clay Science. Elsevier, Amsterdam, pp. 523–557. https://doi.org/10.1016/B978-0-08-098258-8.00017-1
Bergaya F, Aouad A, Mandalia T (2006) Pillared clays and clay minerals. Dev Clay Sci 1:393–421. https://doi.org/10.1016/S1572-4352(05)01012-3
Aouad A, Mandalia T, Bergaya F (2005) A novel method of Al-pillared montmorillonite preparation for potential industrial up-scaling. Appl Clay Sci 28:175–182. https://doi.org/10.1016/j.clay.2004.02.003
Ding M, Zuo S, Qi C (2015) Preparation and characterization of novel composite AlCr-pillared clays and preliminary investigation for benzene adsorption. Appl Clay Sci 115:9–16. https://doi.org/10.1016/j.clay.2015.07.020
Olaya A, Blanco G, Bernal S, Moreno S, Molina R (2009) Synthesis of pillared clays with Al–Fe and Al–Fe–Ce starting from concentrated suspensions of clay using microwaves or ultrasound, and their catalytic activity in the phenol oxidation reaction. Appl Catal B 93:56–65. https://doi.org/10.1016/j.apcatb.2009.09.012
Sivaiah MV, Petit S, Brendlé J, Patrier P (2010) Rapid synthesis of aluminium polycations by microwave assisted hydrolysis of aluminium via decomposition of urea and preparation of Al-pillared montmorillonite. Appl Clay Sci 48:138–145. https://doi.org/10.1016/j.clay.2009.11.016
Sanabria NR, Molina R, Moreno S (2009) Effect of Ultrasound on the structural and textural properties of Al–Fe pillared clays in a concentrated medium. Catal Lett 130:664. https://doi.org/10.1007/s10562-009-9956-4
Rahmani F, Haghighi M, Estifaee P (2014) Synthesis and characterization of Pt/Al2O3–CeO2 nanocatalyst used for toluene abatement from waste gas streams at low temperature: Conventional vs. plasma–ultrasound hybrid synthesis methods. Microporous Mesoporous Mater 185:213–223. https://doi.org/10.1016/j.micromeso.2013.11.019
Duong LV, Kloprogge JT, Frost RL, van Veen JAR (2007) An improved route for the synthesis of Al13-pillared montmorillonite catalysts. J Porous Mater 14:71–79. https://doi.org/10.1007/s10934-006-9010-5
Tomul F (2016) The effect of ultrasonic treatment on iron–chromium pillared bentonite synthesis and catalytic wet peroxide oxidation of phenol. Appl Clay Sci 120:121–134. https://doi.org/10.1016/j.clay.2015.11.007
Baloyi J, Ntho T, Moma J (2018) Synthesis and application of pillared clay heterogeneous catalysts for wastewater treatment: a review. RSC Adv 8:5197–5211. https://doi.org/10.1039/C7RA12924F
Gil SA, Korili R, Trujillano MA, Vicente (2011) A review on characterization of pillared clays by specific techniques. Appl Clay Sci 53:97–105. https://doi.org/10.1016/j.clay.2010.09.018
Mata G, Trujillano R, Vicente MA, Belver C, Fernández-García M, Korili SA, Gil A (2007) Chromium-saponite clay catalysts: Preparation, characterization and catalytic performance in propene oxidation. Appl Catal A 327:1–12. https://doi.org/10.1016/j.apcata.2007.04.021
Corporation GM (1977) United States Patent 19
De León MA, Santos CDL, Latrónica L, Cesio AM, Volzone C, Castiglioni J, Sergio M (2014) High catalytic activity at low temperature in oxidative dehydrogenation of propane with Cr–Al pillared clay. Chem Eng J 241:336–343. https://doi.org/10.1016/j.cej.2013.10.086
Cañizares P, Valverde JL, Kou MRS, Molina CB (1999) Synthesis and characterization of PILCs with single and mixed oxide pillars prepared from two different bentonites. A comparative study. Microporous Mesoporous Mater 29:267–281. https://doi.org/10.1016/S1387-1811(98)00295-9
Gągol M, Przyjazny A, Boczkaj G (2018) Wastewater treatment by means of advanced oxidation processes based on cavitation: a review. Chem Eng J 338:599–627. https://doi.org/10.1016/j.cej.2018.01.049
Baloyi J, Ntho T, Moma J (2018) Synthesis of highly active and stable Al/Zr pillared clay as catalyst for catalytic wet oxidation of phenol. J Porous Mater. https://doi.org/10.1007/s10934-018-0667-3
Tomul F (2012) Adsorption and catalytic properties of Fe/Cr-pillared bentonites. Chem Eng J. https://doi.org/10.1016/j.cej.2012.01.094
Tomul F (2011) Synthesis, characterization, and adsorption properties of Fe/Cr-pillared bentonites. Ind Eng Chem Res 50:7228–7240. https://doi.org/10.1021/ie102073v
Tepmatee P, Siriphannon P (2013) Effect of preparation method on structure and adsorption capacity of aluminum pillared montmorillonite. Mater Res Bull 48:4856–4866. https://doi.org/10.1016/j.materresbull.2013.06.066
Tomul F, Balci S (2009) Characterization of Al, Cr-pillared clays and CO oxidation. Appl Clay Sci 43:13–20. https://doi.org/10.1016/j.clay.2008.07.006
Grim RE (1968) Clay mineralogy. McGraw-Hill, New York, p 596
Vhahangwele M, Mugera GW (2015) The potential of ball-milled South African bentonite clay for attenuation of heavy metals from acidic wastewaters: simultaneous sorption of Co2+, Cu2+, Ni2+, Pb2+, and Zn2+ ions. J Environ Chem Eng 3:2416–2425. https://doi.org/10.1016/j.jece.2015.08.016
Masindi V, Gitari MW, Tutu H, DeBeer M (2015) Efficiency of ball milled South African bentonite clay for remediation of acid mine drainage. J Water Process Eng 8:227–240. https://doi.org/10.1016/j.jwpe.2015.11.001
Gu L, Xu J, Lv L, Liu B, Zhang H, Yu X, Luo Z (2011) Dissolved organic nitrogen (DON) adsorption by using Al-pillared bentonite. Desalination 269:206–213. https://doi.org/10.1016/j.desal.2010.10.063
Huang W, Chen J, He F, Tang J, Li D, Zhu Y, Zhang Y (2015) Effective phosphate adsorption by Zr/Al-pillared montmorillonite: insight into equilibrium, kinetics and thermodynamics. Appl Clay Sci 104:252–260. https://doi.org/10.1016/j.clay.2014.12.002
Li K, Lei J, Yuan G, Weerachanchai P, Wang J-Y, Zhao J, Yang Y (2017) Fe-, Ti-, Zr- and Al-pillared clays for efficient catalytic pyrolysis of mixed plastics. Chem Eng J 317:800–809. https://doi.org/10.1016/j.cej.2017.02.113
Chen D, Cen C, Feng J, Yao C, Li W, Tian S, Xiong Y (2016) Co-catalytic effect of Al-Cr pillared montmorillonite as a new SCR catalytic support. J Chem Technol Biotechnol 91:2842–2851. https://doi.org/10.1002/jctb.4899
Bel Hadjltaief H, Da Costa P, Galvez ME, Ben Zina M (2013) Influence of operational parameters in the heterogeneous photo-Fenton discoloration of wastewaters in the presence of an iron-pillared clay. Ind Eng Chem Res 52:16656–16665. https://doi.org/10.1021/ie4018258
Kloprogge JT (2017) Infrared and raman spectroscopies of pillared clays. In: Gates WP, Kloprogge JT, Madejová J, Bergaya F (eds.) Infrared Raman spectroscopy of clay minerals. Elsevier, Amsterdam, pp 411–446. https://doi.org/10.1016/B978-0-08-100355-8.00012-6
Rocha MAL, Del G, Ángel G, Torres-Torres A, Cervantes A, Vázquez A, Arrieta JN, Beltramini (2015) Effect of the Pt oxidation state and Ce3+/Ce4+ ratio on the Pt/TiO2-CeO2 catalysts in the phenol degradation by catalytic wet air oxidation (CWAO). Catal Today 250:145–154. https://doi.org/10.1016/j.cattod.2014.09.016
Arena F, Negro J, Parmaliana A, Spadaro L, Trunfio G (2007) Improved MnCeOx systems for the catalytic wet oxidation (CWO) of phenol in wastewater streams. Ind Eng Chem Res 46:6724–6731. https://doi.org/10.1021/ie0701118
Yang S, Zhu W, Wang J, Chen Z (2008) Catalytic wet air oxidation of phenol over CeO2-TiO2 catalyst in the batch reactor and the packed-bed reactor. J Hazard Mater 153:1248–1253. https://doi.org/10.1016/j.jhazmat.2007.09.084
Yang S, Cui Y, Sun Y, Yang H (2014) Graphene oxide as an effective catalyst for wet air oxidation of phenol. J Hazard Mater 280:55–62. https://doi.org/10.1016/j.jhazmat.2014.07.051
Davies D, Golunski S, Johnston P, Lalev G, Taylor SH (2018) Dominant effect of support wettability on the reaction pathway for catalytic wet air oxidation over Pt and Ru nanoparticle catalysts. ACS Catal 8:2730–2734. https://doi.org/10.1021/acscatal.7b04039
Delgado JJ, Chen X, Pérez-Omil JA, Rodríguez-Izquierdo JM, Cauqui MA (2012) The effect of reaction conditions on the apparent deactivation of Ce–Zr mixed oxides for the catalytic wet oxidation of phenol. Catal Today 180:25–33. https://doi.org/10.1016/j.cattod.2011.03.069
Zapico RR, Marín P, Díez FV, Ordóñez S (2017) Assessment of phenol wet oxidation on CuO/γ-Al2O3 catalysts: competition between heterogeneous and leached-copper homogeneous reaction paths. J Environ Chem Eng 5:2570–2578. https://doi.org/10.1016/j.jece.2017.04.050
Guo J, Al-Dahhan M (2005) Modeling catalytic trickle-bed and upflow packed-bed reactors for wet air oxidation of phenol with phase change. Ind Eng Chem Res 44 (17): 6634–6642. https://doi.org/10.1021/ie050335v
Álvarez PM, McLurgh D (2002) Plucinski, copper oxide mounted on activated carbon as catalyst for wet air oxidation of aqueous phenol. 1. Kinetic and mechanistic approaches. Ind Eng Chem Res 41:2147–2152. https://doi.org/10.1021/ie0104464
Quintanilla A, Menéndez N, Tornero J, Casas JA, Rodríguez JJ (2008) Surface modification of carbon-supported iron catalyst during the wet air oxidation of phenol: Influence on activity, selectivity and stability. Appl Catal B 81:105–114. https://doi.org/10.1016/j.apcatb.2007.11.034
Arena F, Italiano C, Spadaro L (2012) Efficiency and reactivity pattern of ceria-based noble metal and transition metal-oxide catalysts in the wet air oxidation of phenol, Appl Catal B. https://doi.org/10.1016/j.apcatb.2011.12.035
Santos A, Yustos P, Quintanilla A, Rodríguez S, García-Ochoa F (2002) Route of the catalytic oxidation of phenol in aqueous phase. Appl Catal B 39:97–113. https://doi.org/10.1016/S0926-3373(02)00087-5
Zazo JA, Casas JA, Mohedano AF, Gilarranz MA, Rodríguez JJ (2005) Chemical pathway and kinetics of phenol oxidation by Fenton’s reagent. Environ Sci Technol 39:9295–9302. https://doi.org/10.1021/es050452h
Lal K, Garg A (2015) Catalytic wet oxidation of phenol under mild operating conditions: development of reaction pathway and sludge characterization. Clean Technol Environ Policy 17:199–210. https://doi.org/10.1007/s10098-014-0777-9
Acknowledgements
We kindly acknowledge the financial support of the National Research Foundation (NRF)/ Department of Science and Technology (DST) of South Africa under Professional Development Programme (PDP) Project No. 96719 and Mineral Science Council of South Africa (Mintek) under CWO of Wastewater Project No. ADE 164.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Baloyi, J., Ntho, T. & Moma, J. A Novel Synthesis Method of Al/Cr Pillared Clay and its Application in the Catalytic Wet Air Oxidation of Phenol. Catal Lett 148, 3655–3668 (2018). https://doi.org/10.1007/s10562-018-2579-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10562-018-2579-x