Abstract
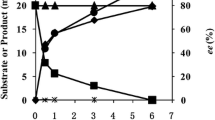
d-Amino acids are pharmaceutically important building blocks, leading to a great deal of research efforts to develop cost-effective synthetic methods. Preparation of d-amino acids by deracemization has been conceptually attractive owing to facile synthesis of racemic amino acids by Strecker synthesis. Here, we demonstrated biocatalytic deracemization of aliphatic amino acids into d-enantiomers by running cascade reactions; (1) stereoinversion of l-amino acid to a d-form by amino acid dehydrogenase and ω-transaminase and (2) regeneration of NAD+ by NADH oxidase. Under the cascade reaction conditions containing 100 mM isopropylamine and 1 mM NAD+, complete deracemization of 100 mM dl-alanine was achieved after 24 h with 95% reaction yield of d-alanine (> 99% eeD, 52% isolation yield).
Graphical Abstract





Similar content being viewed by others
References
Fuchs SA, Berger R, Klomp LW, de Koning TJ (2005) Mol Genet Metab 85:168–180
Kiriyama Y, Nochi H (2016) Scientifica (Cairo) 2016:6494621
Genchi G (2017) Amino Acids 49:1521–1533
Gao X, Ma Q, Zhu H (2015) Appl Microbiol Biotechnol 99:3341–3349
Martinez-Rodriguez S, Martinez-Gomez AI, Rodriguez-Vico F, Clemente-Jimenez JM, Heras-Vazquez LFJ (2010) Chem Biodiv 7:1531–1548
Tsai G, Coyle J (2001) US Patent 6,228,875 B1
Huirne JA, Lambalk CB (2001) Lancet 358:1793–1803
Yuasa Y, Nagakura A, Tsuruta H (2001) J Agric Food Chem 49:5013–5018
Ma JA (2003) Angew Chem Int Ed 42:4290–4299
Breuer M, Ditrich K, Habicher T, Hauer B, Kesseler M, Sturmer R, Zelinski T (2004) Angew Chem Int Ed 43:788–824
Xue Y-P, Cao C-H, Zheng Y-G (2018) Chem Soc Rev 47:1516–1561
Vedha-Peters K, Gunawardana M, Rozzell JD, Novick SJ (2006) J Am Chem Soc 128:10923–10929
Ager DJ, Fotheringham IG (2001) Curr Opin Drug Discov Dev 4:800–807
Park ES, Dong JY, Shin JS (2013) Org Biomol Chem 11:6929–6933
Park ES, Dong JY, Shin JS (2013) ChemCatChem 5:3538–3542
Park ES, Dong JY, Shin JS (2014) Appl Microbiol Biotechnol 98:651–660
Merino P, Marqués-López E, Tejero T, Herrera RP (2009) Tetrahedron 65:1219–1234
Yano S, Haruta H, Ikeda T, Kikuchi T, Murakami M, Moriguchi M, Wakayama M (2011) J Chromatogr B 879:3247–3252
Carboni C, Gardossi L, Tamiola K, Janssen DB, Quaedflieg PJLM (2006) Tetrahedron Asymmetry 17:245–251
Krieg L, Ansorge-Schumacher MB, Kula MR (2002) Adv Synth Catal 344:965–973
Isobe K, Tamauchi H, Fuhshuku K, Nagasawa S, Asano Y (2010) Enzyme Res 2010:567210
Wegman MA, Janssen MHA, van Rantwijk F, Sheldon RA (2001) Adv Synth Catal 343:559–576
Servi S, Tessaro D, Pedrocchi-Fantoni G (2008) Coord Chem Rev 252:715–726
Park ES, Shin JS (2014) Adv Synth Catal 356:3505–3509
Schatzle S, Höhne M, Redestad E, Robins K, Bornscheuer UT (2009) Anal Chem 81:8244–8248
Bhushan R, Bruckner H (2004) Amino Acids 27:231–247
Malik MS, Park ES, Shin JS (2012) Appl Microbiol Biotechnol 94:1163–1171
Seo YM, Mathew S, Bea HS, Khang YH, Lee SH, Kim BG, Yun H (2012) Org Biomol Chem 10:2482–2485
Geueke B, Riebel B, Hummel W (2003) Enzyme Microb Technol 32:205–211
Acknowledgements
This work was funded by the National Research Foundation of Korea under the Basic Science Research Program (2016R1A2B400840). We thank Mrs Sae-Rom Park for technical assistance in the cloning of NOX.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors declare that they have no conflict of interest to disclose.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Han, SW., Shin, JS. One-Pot Preparation of d-Amino Acids Through Biocatalytic Deracemization Using Alanine Dehydrogenase and ω-Transaminase. Catal Lett 148, 3678–3684 (2018). https://doi.org/10.1007/s10562-018-2565-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10562-018-2565-3