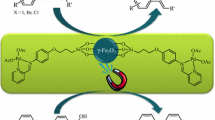
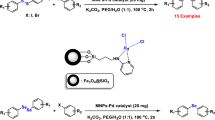
Abstract
Synthesis and characterization of new magnetically recoverable and high-performance nanocatalysts of Pd and Pt Schiff base complexes on magnetite nanoparticles were considered. Catalytic activity of these nanocatalysts was explored in Suzuki and Heck cross coupling reactions. The catalysts can be easily reused fourth consecutive runs without significant loss of catalytic efficiency.
Graphical Abstract









Similar content being viewed by others
References
Abu-Surrah AS, Abu Safieh KA, Ahmad IM, Abdalla MY, Ayoub MT, Qaroush AK, Abu-Mahtheieh AM (2010) Eur J Med Chem 45:471
Chandra S, Saneetika X (2004) Spectr Chem Acta A 60:147
Saghatforoush LA, Aminkhani A, Ershad S, Karimnezhad G, Ghammamy S, Kabiri R (2008) Molecules 13:804
Cozzi PG (2004) Chem Soc Rev 33:410
Khorrami AR, Naeimi H, Fakhari AR (2004) Talanta 64:13
Ardakany MM, Ensafi AA, Naeimi H, Dastanpour A, Shamlli A (2003) Sens Actuators B Chem 96:441
Bedford RB, Hazelwood SL, Limmert ME (2002) Chem Commun 2610
Tong J, Li Z, Xia C (2005) J Mol Catal A Chem 231:197
Cavazzini M, Pozzi G, Quici S, Shepperson I (2003) J Mol Catal A Chem 433:204
Murahashi SI, Noji S, Komiya N (2004) Adv Synth Catal 346:195
Parrilha GL, Ferreira SS, Fernandes C, Silva GC, Carvalho NMF, Antunes OAC, Drago V, Bortoluzzi AJ, Horn A Jr (2010) J Br Chem Soc 21:603
Jana R, Pathak TP, Sigman MS (2011) Chem Rev 111:1417
Yeung CS, Dong VM (2011) Chem Rev 111:1215
Chen FR, Huang MM, Li YQ (2014) Ind Eng Chem Res 53:8339
Baudoin O, Cesario M, Guenard D, Gueritte F (2002) J Org Chem 67:1199
Martin R, Buchwald SL (2008) Acc Chem Res 41:1461
Eseola AO, Görls H, Woods JAO, Plass W (2015) J Mol Catal A 406:224
Eseola AO, Akogun O, Görls H, Atolani O, Kolawole GA, Plass W (2014) J Mol Catal A 387:112
Eseola AO, Geibig D, Görls H, Sun WH, Hao X, Woods JAO, Plass W (2014) J Organomet Chem 754:39
Liu P, Feng XJ, He R (2010) Tetrahedron 66:31
Nasifa S, Biplab B, Pankaj D (2013) Tetrahedron Lett 54:2886
Böhm VPW, Gstöttmayr CWK, Weskamp T, Herrmann WA (2000) J Organomet Chem 595:186
Mobinikhaledi A, Khajeh-Amiri A (2014) Reac Kinet Mech Catal 112:131
Bezaatpoura A, Askarizadehb E, Akbarpoura SH, Amiria M, Babaei B (2017) Mol Catal 436:199
Maleki A, Rahimi R, Malek S (2016) Environ Chem Lett 14:195
Anuradha A, Kumari S, Layek S, Pathak DD (2017) New J Chem 41:5595
Hajjami M, Gholamian F (2016) RSC Adv 6:87950
Ying A, Qiu F, Wu Ch, Hu H, Yang J (2014) RSC Adv 4:33175
Ghorbani-Choghamarani A, Darvishnejad Z, Tahmasbi B (2015) Inorg Chim Acta 435:223
Cho SD, Kim HK, Yim HS, Kim MR, Lee JK, Kim JJ, Yoon YJ (2007) Tetrahedron 63:1345
Alacid E, Nájera C (2008) Org Lett 10:5011
Masllorens J, Gonzalez I, Roglans A (2007) Eur J Org Chem 1:158
Stevens PD, Fan J, Gardimalla HMR, Yen M, Gao Y (2005) Org Lett 7:2085
Lü B, Fu C, Ma S (2010) Tetrahedron Lett 51:1284
Emmanuvel L, Sudalai A (2007) Arkivoc 14:126
Samarasimhars Eddy M, Prabhu G, Vishwanatha TM, Sureshbabu VV (2013) Synthesis 45:1201
Ramesh Kumar NSC, Victor Paul Raj I, Sudalai A (2007) J Mol Catal A Chem 269:218
Baia L, Wanga JX (2007) Adv Synth Catal 350:315
Shah D, Kaur H (2012) J Mol Catal A Chem 359:69
Xu Q, Duan WL, Lei ZY, Zhu ZB, Shi M (2005) Tetrahedron 61:11225
Aksın Ö, Türkmen H, Artok L, Òªetinkaya B, Ni Ch, Büyükgüngör O, Özkal E (2006) J Organomet Chem 691:3027
Youn SW, Song HS, Park JH (2014) Org Biomol Chem 12:2388
Xu HJ, Zhao YQ, Zhou XF (2011) J Org Chem 76:8036
Sugihara T, Satoh T, Miura M, Nomura M (2004) Adv Synth Catal 346:1765
Ma H, Cao W, Bao ZH, Lei Z (2012) Catal Sci Technol 2:2291
Chen W, Zhong L, Peng X, Wang K, Chena ZH, Sun R (2014) Catal Sci Technol 4:1426
Salam N, Kundu SK, Roy AS, Mondal P, Ghosh K, Bhaumik A, Islam SM (2014) Dalton Trans 43:7057
Wang D, Liu W, Bian F, Yu W (2015) New J Chem 39:2052
Sobhani S, Ghasemzadeh MS, Honarmand M, Zarifi F (2014) RSC Adv 4:44166
Yang J, Wang D, Liu W, Zhang X, Bian F, Yu W (2013) Green Chem 15:3429
Sarkar SM, Rahman ML, Yusoff MM (2015) New J Chem 39:3564
Tamami B, Farjadian F, Ghasemi S, Allahyari H (2013) New J Chem 37:2011
Shelkar RS, Gund SH, Nagarkar JM (2014) RSC Adv 4:53387
Shang N, Gao S, Zhou X, Feng C, Wang Z, Wang C (2014) RSC Adv 4:54487
Søbjerg LS, Gauthier D, Lindhardt AT, Bunge M, Finster K, Meyer RL, Skrydstrup T (2009) Green Chem 11:2041
Fakhri A, Naghipour A (2016) Mater Technol 13:846
Acknowledgements
The authors appreciatively acknowledge the funding support received from the Ilam University, Ilam, Iran, on this work.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rezaei, G., Naghipour, A. & Fakhri, A. Catalytic Performance Studies of New Pd and Pt Schiff Base Complexes Covalently Immobilized on Magnetite Nanoparticles as the Environmentally Friendly and Magnetically Recoverable Nanocatalyst in C–C Cross Coupling Reactions. Catal Lett 148, 732–744 (2018). https://doi.org/10.1007/s10562-017-2270-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10562-017-2270-7