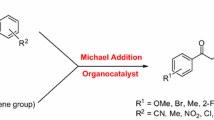
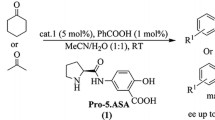
Abstract
In this study, a new organocatalyst derived from proline was developed and shown to be an efficient catalyst for asymmetric Michael addition reactions of ketones and aldehydes to nitroolefins with high diastereo- and enantioselectivities. (syn;anti up to 99:1, ee. up to 98 %.). Furthermore, the catalyst is easily recovered and could be reused six times without significant loss of its ability to affect the outcome of the asymmetric reactions. In addition, computational studies at the B3LYP/6-311G(d,p)//6-311 + G(2dp,f) level was conducted on a model reaction, and confirmed the following hypotheses: first, the hydrogen bonding between carboxyl group and nitro group plays an important role in catalysis, and second, the energy barrier for re-face attack in reactions of ketones to form 2S, 3R products is lower than that for the si-face attack leading to 2S, 3R products.
Graphical Abstract
Structural modification of a previously reported organocatalyst (lead compound in figure) was used to design an efficient and recyclable organocatalyst for asymmetric Michael addition. The introduced carboxyl group not only enhances the enantioselectivity but also brings convenience to the recovery of the catalyst.





Similar content being viewed by others
References
Dalko PI (2004) Angew Chem Int Ed 43:5138
Guillena G, Ramon DJ (2006) Tetrahedron Asymmetry 17:1465
Dalko PI (2007) Enantioselective rganocatalysis. Wiley, Weinheim
List B (2007) Chem Rev 107:5413
Rongming W, Linhai J, Dabin Q (2015) Tetrahedron Lett 56:2867
Masanori Y, Yuki N, Ami K, Shoji H, Masahiro Y (2013) Tetrahedron 69:10003
Clarke ML, Fuentes JA (2007) Angew Chem Int Ed 46:930
Almaşi D, Alonso DA, Gómez-Bengoa E, Nagel Y, Nájera C (2007) Eur J Org Chem 2007:2328
Diez D, Gil MJ, Moro RF, Marcos IS, García P, Basabe P, Garrido NM, Broughton HB, Urones JG (2007) Tetrahedron 63:740
Lu A, Wu R, Wang Y, Zhou Z, Wu G, Fang J, Tang C (2011) Eur J Org Chem 122:3507
Berner OM, Tedeschi L, Enders D (2002) Eur J Org Chem 12:1877
Krause N, Hoffmann-Röder A (2001) Synthesis 1:171
Tsogoeva SB (2007) Eur J Org Chem 11:1701
Almasi D, Alonso DA, Nájera C (2007) Tetrahedron Asymmetry 18:299
Cao CL, Ye MC, Sun XL, Tang Y (2006) Org Lett 8:2901
Freund M, Schenker S, Tsogoeva SB (2009) Org Biomol Chem 7:4279
Zu L, Wang J, Li H, Wang W (2006) Org Lett 8:3077
Lu AD, Gao P, Wu Y, Wang YM, Zhou ZH, Tang CC (2009) Org Biomol Chem 7:3141
Wang J, Li H, Lou B, Zu L, Guo H, Wang W (2006) Chem Eur J 12:4321
Lu A, Wu R, Wang Y, Zhou Z, Wu G, Fang J, Tang C (2010) Eur J Org Chem 2010:2057
Chen JR, Lai YY, Lu HH, Wang XF (2009) Tetrahedron 65:9238
Cao YJ, Lu HH, Lai YY, Lu LQ, Xiao WJ (2006) Synthesis 22:3795
List B, Pojarliev P, Martin HJ (2001) Org Lett 3:2423
Betancort JM, Barbas CF III (2001) Org Lett 3:3737
Enders D, Seki A (2002) Synlett 1:26
Ishii T, Fujioka S, Sekiguchi Y, Kotsuki H (2004) J Am Chem Soc 126:9558
Mase N, Thayumanavan R, Tanaka F, Barbas CFIII (2004) Org Lett 6:2527
Betancort JM, Sakthivel K, Thayumanavan R, Tanaka F, Barbas CFIII (2004) Synthesis 9:1509
Alexakis A, Andrey O (2002) Org Lett 4:3611
Andrey O, Alexakis A, Tomassini A, Bernardinelli G (2004) Adv Synth Catal 346:1147
Cobb AJA, Longbottom DA, Shaw DM, Ley SV (2004) Chem Commun 16:1808
Cobb AJA, Shaw DM, Longbottom DA, Gold JB, Ley SV (2005) Org Biomol Chem 3:84
Reyes E, Vicario JL, Badia D, Carrillo L (2006) Org Lett 8:6135
Wang W, Wang J, Li H (2005) Angew Chem Int Ed 44:1369
Pansare SV, Pandya K (2006) J Am Chem Soc 128:9624
Mase N, Watanabe K, Yoda H, Takabe K, Tanaka F, Barbas CFIII (2006) J Am Chem Soc 128:4966
Vishnumaya, Singh VK (2007) Org Lett 9:1117
Gua L, Zhao G (2007) Adv Synth Catal 349:1629
Ni B, Zhang Q, Headley AD (2007) Tetrahedron Asymmetry 18:1443
Xu DQ, Wang LP, Luo SP, Wang YF, Zhang S, Xu ZY (2008) Eur J Org Chem 6:1049
Bukuo N, Zhang QY, Kritanjali D, Allan DH (2009) Org Lett 11:1037
Diana A, Diego AA, Enrique GB, Yvonne N, Carmen N (2007) Eur J Org Chem 14:2328
Terakado D, Takano M, Oriyama T (2005) Chem Lett 34:962
Wang L, Liu J, Miao T, Zhou W, Li P, Ren K, Zhang X (2010) Adv Synth Catal 352:1629
Zh W, Lu CF, Ch Yang G, Chen ZX, Nie JQ (2015) Catal Commun 62:34
Han Y, Mouming L, Sheng H (2014) Tetrahedron 70:8380
Cao YJ, Lai YY, Wang X, Li YJ, Xiao WJ (2007) Tetrahedron Lett 48:21
Ni B, Zhang Q, Headley AD (2007) Green Chem 9:737
Zhang Q, Ni B, Headley AD (2008) Tetrahedron 64:5091
Wu LY, Yan ZY, Xie YX, Niu YN, Liang YM (2007) Tetradedron Asymmetry 18:2086
Luo S, Mi X, Zhang L, Liu S, Xu H, Cheng JP (2006) Angew Chem Int Ed 45:3093
Alza E, Cambeiro XC, Jimeno C, Pericàs MA (2007) Org Lett 9:3717
Wang B-G, Ma B-C, Wang Q, Wang W (2010) Adv Synth Catal 352:2923
Zheng Z, Perkins BL, Ni B (2010) J Am Chem Soc 132:50
Xin H, Wen-Bin Y, Danash A, Wei Z (2013) Tetrahedron Lett 54:6064
Xuefei Q, Jun T, Yang L, Ligong Ch, Xilong Y (2015) Catal Commun 71:70
Cao X, Wang G, Zhang R, Wei Y, Wang W, Sun H, Chen L (2011) Org Biomol Chem 9:6487
Gaussian 09, Revision D.01, Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Scalmani G, Barone V, Mennucci B, Petersson GA, Nakatsuji H, Caricato M, Li X, Hratchian HP, Izmaylov AF, Bloino J, Zheng G, Sonnenberg JL, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Vreven T, Montgomery JA Jr., Peralta JE, Ogliaro F, Bearpark M, Heyd JJ, Brothers E, Kudin KN, Staroverov VN, Keith T, Kobayashi R, Normand J, Raghavachari K, Rendell A, Burant JC, Iyengar SS, Tomasi J, Cossi M, Rega N, Millam JM, Klene M, Knox JE, Cross JB, Bakken V, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Martin RL, Morokuma K, Zakrzewski VG, Voth GA, Salvador P, Dannenberg JJ, Dapprich S, Daniels AD, Farkas O, Foresman JB, Ortiz JV, Cioslowski J, Fox DJ (2013) Gaussian, Inc., Wallingford CT
Becke AD (1993) J Chem Phys 98:1372
Becke AD (1993) J Chem Phys 98:5648
Lee C, Yang W, Parr RG (1988) Phys Rev B 37:785
Gonzalez C, Schlegel HB (1990) J Phys Chem 94:5523
Knudsen RK, Mitchell CET, Ley SV (2006) Chem Commun 1:66
Tang Z, Yang Z, Chen X, Cun L, Mi A, Jiang Y, Gong L (2005) J Am Chem Soc 127:9285
Martin HJ, List B (2003) Synlett 12:1901
Lu D, Gong Y, Wang W (2010) Adv Synth Catal 352:644
Asami M (1990) Bull Chem Sc Jpn 63:721
Carter ME, Nash JL Jr, Drueke JW Jr, Schwietert JW, Butler GB (1978) J Polym Sci Polym Chem Ed 16:937
Mase N, Tanaka F, Barbas CFIII (2003) Org Lett 5:4369
Acknowledgments
This work was supported by Supported by National Natural Science Foundation of China (No. 21306038) and Natural Science Foundation of Hebei Province (No. B2013202216).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Yang, M., Zhang, Y., Zhao, J. et al. A Recyclable Organocatalyst for Asymmetric Michael Addition. Catal Lett 146, 587–595 (2016). https://doi.org/10.1007/s10562-016-1693-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10562-016-1693-x