Abstract
Quantitative description for turnover frequency dependence on the metal cluster size is discussed for competitive Langmuir–Hinshelwood mechanism showing that the apparent reaction orders depend on the cluster size. Position of the maximum rate of the turnover frequency for a two-step sequence is a function on temperature and only in a special case, maximum of the turnover frequency is temperature independent on reaction temperature. For the same reaction mechanism the impact of internal diffusion limitations on structure sensitivity is discussed.
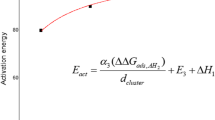
Graphical Abstract



Similar content being viewed by others
References
van Hardeveld R, Hartog F (1969) Surf Sci 15:189
Bell AT (2003) Science 299:1688
Schlögl R, Abd SB (2004) Hamid. Angew Chem Int Ed 43:1628
Narayanan R, El-Sayed MA (2008) Top Catal 47:15
Klasovsky F, Claus P (2008) In: Corain B, Schmid G, Toshima N (eds) Metal nanoclusters in catalysis and materials science: the issue of size control. Elsevier, Amsterdam, pp 167–181
Santen RA (2009) Acc Chem Res 42:57
Che M, Bennett CO (1989) Adv Catal 36:55
Henry CR (2000) Appl Surf Sci 164:252
Boudart M (1969) Adv Catal 20:153
Murzin DYu (2012) Catal Lett 142:1279
Murzin DYu (2009) Chem Eng Sci 64:64
Murzin DYu (2010) J Mol Catal A 315:226
Murzin DYu, Parmon VN (2011) Catal-Spec Period Rep, RSC 23:179
Parmon VN (2007) Dokl Phys Chem 413:42
Murzin DYu (2010) J Catal 276:85
Simakova O, Kusema B, Campo B, Leino A-R, Kordas K, Pitchon V, Mäki-Arvela P, Murzin DYu (1036) J Phys Chem C 2011:115
Simakova OA, Murzina EV, Murzin DYu (2014) C R Chim 17:770
Aho A, Roggan S, Simakova O, Salmi T, Murzin DYu (2015) Catal Today 241:195
Baeza JA, Calvo L, Murzin DYu, Rodriguez JJ, Gillaranz MA (2014) Catal Lett 144:2080
Murzin DYu, Simakova IL (2010) Kinet Catal 51:828
Murzin DYu (2014) Catal Sci Technol 4:3340
Boudart M (1968) Kinetics of chemical processes. Prentice-Hall, Englewood Cliffs
Salmi TO, Mikkola J-P, Wärnå JP (2009) Chemical reaction engineering and reactor technology. CRC, Boca Raton
Murzin D, Salmi T (2005) Catalytic kinetics. Elsevier, Amsterdam
Temkin MI (1984) Kinet Catal 25:299
Rioux RM, Hsu BB, Grass ME, Song H, Somorjai GA (2008) Catal Lett 126:10
Parmon VN (2015) Thermodynamics of irreversible processes for chemists, Intellect, ISBN: 978-5-91559-189-8, Moscow
Murzin DYu (2013) Engineering catalysis. De Gruyter, Berlin
Beck IE, Bukhtiyarov VI, Pakharukov IYu, Zaikovsky VI, Kriventsov VV, Parmon VN (2009) J Catal 268:60
Chen J, Zhang Q, Wang Y, Wan H (2008) Adv Synth Catal 350:453
Acknowledgments
This work was partially executed at the Laboratory of Catalytic Technologies at St. Petersburg State Institute of Technology supported by the mega-grant of the Government of the Russian Federation.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Murzin, D.Y. Cluster Size Dependent Kinetics: Analysis of Different Reaction Mechanisms. Catal Lett 145, 1948–1954 (2015). https://doi.org/10.1007/s10562-015-1604-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10562-015-1604-6