Abstract
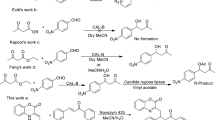
Lipases catalyzed the reaction between 4-nitrobenzaldehyde and 2-cyclohexen-1-one in aqueous-DMSO co-solvent mixtures to give Morita–Baylis–Hillman product and aldol product. Among lipases, Burkholderia cepacia lipase gave the best overall conversion of 96 % in 50 % (v/v) DMSO while Mucor javanicus lipase showed highest stereoselectivity in the formation of the aldol (79 % ee) and Morita–Baylis–Hillman product (63 % ee) with 30 % (v/v) DMSO.
Graphical Abstract
Lipase catalysed Morita–Baylis–Hillman reaction




Similar content being viewed by others
References
Singh A, Chisti Y, Banerjee UC (2012) Process Biochem 47:2398–2404
Othman SS, Basri M, Hussein MZ, Rahman MBA, Jasmani H, Rahman RNZRA, Salleh AB (2008) Food Chem 106:437–443
Rahman MBA, Zaidan US, Basri M, Salleh AB, Rahman RNZRA, Hussein MZ (2008) J Mol Catal B: Enzym 50:33–39
Adlercreutz P (2013) Chem Soc Rev 42:6406–6436
Gotor V, Alfonso I, Uradiales EG (2008) Asymmetric organic synthesis with enzymes. Wiley-VCH Verlag, Weinheim
Hult K, Berglund P (1987) Trends Biotechnol 25:231–238
Yang F, Wang Z, Wang H, Zhang H, Yue H, Wang L (2014) R Soc Chem Adv 4:25633–25636
Gupta MN, Kapoor M, Majumder AB, Singh V (2011) Curr Sci 100:1152–1163
Kapoor M, Gupta MN (2012) Process Biochem 47:555–569
Majumder A, Gupta MN (2014) Synth Commun 44:818–826
Wang H, Wang Z, Wang C, Yang F, Zhang H, Yue H, Wang L (2014) R Soc Chem Adv 4:35686–35689
Langer P (2000) Angew Chem Int Ed 39:3049–3052
Shi Y-L, Shi M (2007) Eur J Org Chem 18:2905–2916
Basavaiah D, Veeraraghavaiah G (2012) Chem Soc Rev 41:68–78
Reetz MT, Mondiere R, Carballeira JD (2007) Tetrahedron Lett 48:1679–1681
Lopez-Iglesias M, Busto E, Gotor V, Gotor-Fernandes V (2011) Adv Synth Catal 353:2345–2353
Jiang L, Yu H-W (2014) Biotechnol Lett 36:99–103
Li K, He T, Li C, Feng X-W, Wang N, Yu X-Q (2009) Green Chem 11:777–779
de Souza ROMA, Matos LMC, Gonçalves KM, Costa ICR, Babics I, Leite SGF, Oestreicher EG, Antunes OAC (2009) Tetrahedron Lett 50:2017–2018
Arora B, Pandey PS, Gupta MN (2014) Tetrahedron Lett 55:3920–3922
Majumder AB, Ramesh NG, Gupta MN (2009) Tetrahedron Lett 50:5190–5193
Kataoka T, Iwama T, Tsujiyama S-I, Iwamura T, Watanabe S-I (1998) Tetrahedron 54:11813–11824
Luo S, Zhang B, He J, Janezuk A, Wang PG, Cheng J-P (2002) Tetrahedron Lett 43:7361–7369
Wu W-B, Xu J-M, Wu Q, Lv D-S, Lin X-F (2006) Adv Synth Catal 348:487–492
Wang J-L, Liu B-K, Yin C, Wu Q, Lin X-F (2011) Tetrahedron 67:2689–2692
Lanne C (1987) Biocatalysis 30:17–22
Gupta MN (1992) Eur J Biochem 203:25–32
Carrea G, Riva S (2000) Angew Chem Int Ed 39:2226–2254
Khmelnitsky YL, Mozhaev VV, Belova AB, Sergeeva MV, Martinek K (1991) Eur J Biochem 198:31–41
McDougal NT, Trevellini WL, Rodgen SA, Kliman LT, Schaus SE (2004) Adv Synth Catal 346:1231–1240
Svedendahl M, Carlqvist P, Branneby C, Allner O, Frise A, Hult K, Berglund P, Brinck T (2008) ChemBioChem 9:2443–2451
Torre O, Alfonso I, Gotor V (2004) Chem Commun 1724–1725
Carlqvist P, Svedendahl M, Branneby C, Hult K, Brinck T, Berglund P (2005) ChemBioChem 6:331–336
Khersonsky O, Tawfik DS (2010) Ann Rev Biochem 79:471–505
Gatri R, El Gaied MM (2002) Tetrahedron Lett 43:7835–7836
Rastogi N, Namboothiri INN, Cojocaru M (2004) Tetrahedron Lett 45(24):4745–4748
Bjelic S, Nivon LG, Çelebi-Olçum N, Kiss G, Rosewall CF, Lovick HM, Ingalls EL, Gallaher JL, Seetharaman J, Lew S, Montelione GT, Hunt JF, Michael FE, Houk KN, Baker D (2013) ACS Chem Biol 8:749–757
Branneby C, Carlqvist P, Magnusson A, Hult K, Brinck T, Berglund P (2003) J Am Chem Soc 125:874–875
Shi M, Liu X-G (2008) Org Lett 10:1043–1046
Acknowledgments
The work was supported by financial support of the Department of Science and Technology (DST), Govt. of India (Grant No.: SR/SO/BB-68/2010).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Kapoor, M., Majumder, A.B. & Gupta, M.N. Promiscuous Lipase-Catalyzed C–C Bond Formation Reactions Between 4 Nitrobenzaldehyde and 2-Cyclohexen-1-one in Biphasic Medium: Aldol and Morita–Baylis–Hillman Adduct Formations. Catal Lett 145, 527–532 (2015). https://doi.org/10.1007/s10562-014-1429-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10562-014-1429-8