Abstract
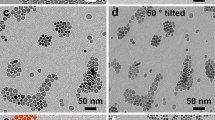
We demonstrate a technique to prepare high surface area ZnO powders that preferentially favor a plate-like morphology, exposing the \((0001)/(000\bar{1})\) facets. A solution-based synthetic route was used to decompose zinc acetate in the presence of amine and citrate ions to block the \((0001)/(000\bar{1})\) facets and favor growth from the pyramid planes. The ZnO platelets remained stable upon heating to 250 °C as evidenced by electron diffraction patterns. The high surface area (75 m2/g) and surface energetics of the (0001) plane make these powders suitable as supports for heterogeneous catalysts.
Graphical Abstract











Similar content being viewed by others
References
Vesborg PCK, Chorkendorff I, Knudsen I, Balmes O, Nerlov J, Molenbroek AM, Clausen BS, Helveg S (2009) J Catal 262(1):65. doi:10.1016/j.jcat.2008.11.028
Iwasa N, Takezawa N (2003). Top Catal V22(3):215. doi:10.1023/A:1023571819211
Karim AM, Conant T, Datye AK (2008) Phys Chem Chem Phys 10:5584. doi:10.1039/b800009c
Cheng WH, Akhter S, Kung HH (1983) J Catal 82(2):341. doi:10.1016/0021-9517(83)90200-2
Wilmer H, Kurtz M, Klementiev KV, Tkachenko OP, Grünert W, Hinrichsen O, Birkner A, Rabe S, Merz K, Driess M, Wöll C, Muhler M (2003) Phys Chem Chem Phys 5:4736. doi:10.1039/b304425d
Vohs J, Barteau M (1986) Surf Sci 176(1–2):91. doi:10.1016/0039-6028(86)90165-2
Hyman MP, Lebarbier VM, Wang Y, Datye AK, Vohs JM (2009) J Phys Chem C 113(17):7251. doi:10.1021/jp809934f
Bowker M, Houghton H, Waugh KC (1981) J Chem Soc Faraday Trans 1: Phys Chem Condens Phases 77:3023. doi:10.1039/F19817703023
Halevi B, Vohs JM (2005) J Phys Chem B 109(50):23976
Diebold U, Koplitz LV, Dulub O (2004) Appl Surf Sci 237(1–4):336
Dulub O, Boatner LA, Diebold U (2002) Surf Sci 519(3):201
Ada K, Gökgöz M, Önal M, Sarıkaya Y (2008) Powder Technol 181(3):285. doi:10.1016/j.powtec.2007.05.015
Li GR, Hu T, Pan GL, Yan TY, Gao XP, Zhu HY (2008) J Phys Chem C 112(31):11859. doi:10.1021/jp8038626
Xu CX, Sun XW, Dong ZL, Yu MB (2004) Appl Phys Lett 85(17):3878. doi:10.1063/1.1811380
Wang F, Liu R, Pan A, Cao L, Cheng K, Xue B, Wang G, Meng Q, Li J, Li Q, Wang Y, Wang T, Zou B (2007) Mater Lett 61(10):2000. doi:10.1016/j.matlet.2006.08.007
Staemmler V, Fink K, Meyer B, Marx D, Kunat M, Girol SG, Burghaus U, Wöll C (2003) Phys Rev Lett 90(10):106102/1
Yu Q, Yu C, Yang H, Fu W, Chang L, Xu J, Wei R, Li H, Zhu H, Li M, Zou G, Wang G, Shao C, Liu Y (2007) Inorg Chem 46(15):6204. doi:10.1021/ic070008a
Wang M, Hahn SH, Kim JS, Chung JS, Kim EJ, Koo KK (2008) J Cryst Growth 310(6):1213. doi:10.1016/j.jcrysgro.2008.01.001
Tian ZR, Voigt JA, Liu J, McKenzie B, McDermott MJ, Rodriguez MA, Konishi H, Xu H (2003) Nat Mater 2(12):821. doi:10.1038/nmat1014
Rietveld HM (1969) J Appl Crystallogr 2(2):65. doi:10.1107/S0021889869006558
Larson AC, Dreele RBV (2004) General structure analysis system (GSAS). Technical report, Los Alamos National Laboratory Report LAUR 86-748
Toby BH (2001) J Appl Crystallogr 34(2):210 doi:10.1107/S0021889801002242
Downs RT, Hall-Wallace M (2003) . Am Mineral 88:247
Brunauer S, Emmett PH, Teller E (1938) J Am Chem Soc 60(2):309
Cho S, Jang JW, Jung SH, Lee BR, Oh E, Lee KH (2009) Langmuir 25(6):3825. doi:10.1021/la804009g
Chippindale AM, Hibble SJ, Bilbé EJ (2009) Acta Crystallogr Sect C 65(7):i39. doi:10.1107/S0108270109020885. http://dx.doi.org/10.1107/S0108270109020885
Abràmoff MD, Magalhães PJ, Ram SJ (2004) Biophotonics Int 11(7):36
Cao X, Zeng H, Wang M, Xu X, Fang M, Ji S, Zhang L (2008) J Phys Chem C 112(14):5267. doi:10.1021/jp800499r
Acknowledgements
This work has been supported by the United States Department of Energy Office of Basic Energy Science, Division of Chemical Sciences under contract number DE-FG02-05ER15712 (University of New Mexico), and DE-AC0494AL85000 (Sandia National Laboratories), Division of Material Science. Sandia is a multiprogram laboratory operated by Sandia Corporation, a Lockheed Martin Company for the United States Department of Energy.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Burton, P.D., Peterson, E.J., Boyle, T.J. et al. Synthesis of High Surface Area ZnO(0001) Plates as Novel Oxide Supports for Heterogeneous Catalysts. Catal Lett 139, 26–32 (2010). https://doi.org/10.1007/s10562-010-0405-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10562-010-0405-1