Abstract
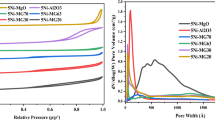
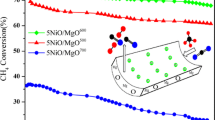
The effect of calcination temperature on the properties of eggshell Ni/MgO–Al2O3 catalysts was studied. Catalyst deactivation was also investigated. It is found that higher calcination temperature contributes to lower surface area, wider pore, and larger Ni crystallites. Small Ni crystallites and large pores favor the catalyst performance. Catalyst deactivation is due to the formation of NiO–MgO solid solution and/or NiAl2O4, phase transformation, and sintering.








Similar content being viewed by others
References
Ashcroft AT, Cheetham AK, Foord JS, Green MLH, Grey CP, Murrell AJ, Vernon PDF (1990) Nature 344:319
Zhang YH, Xiong GX, Sheng SS, Yang WS (2000) Catal Today 63:517
Choudhary VR, Uphade BS, Mamman AS (1995) Catal Lett 32:387
Qiu YJ, Chen JX, Zhang JY (2007) Front Chem Eng China 1:167
Requies J, Cabrero MA, Barrio VL, Cambra JF, Güemez MB, Arias PL, Parola VL, Peña MA, Fierro JLG (2006) Catal Today 116:304
Koo KY, Roh HS, Seo YT, Seo DJ, Yoon WL, Park SB (2008) Int J Hydrogen Energy 33:2036
Koo KY, Roh HS, YTc Seo, Seo DJ, Yoon WL, Park SB (2008) Appl Catal A 340:183
Qiu YJ, Chen JX, Zhang JY React Kinet Catal Lett (accepted for publication)
Hickman DH, Schimdt LD (1992) J Catal 138:267
Choudary VR, Mamman AS, Sansare SD (1992) Angew Chem Int Ed Engl 31:1189
Qiu YJ, Chen JX, Zhang JY (2007) Catal Commun 8:508
Song YQ, He DH, Xu BQ (2008) Appl Catal A 337:19
Utaka T, Al-Drees SA, Ueda J, Iwasa Y, Takeguchi T, Kikuchi R, Eguchi K (2003) Appl Catal A 247:125
Andryushkova OV, Kirichenko OA, Ushakov VA, Poluboyarov VA (1997) Solid State Ion 101–103:647
Johnson MFL (1990) J Catal 123:245
Burtin P, Brunelle JP, Pijolat M, Soustelle M (1987) Appl Catal 34:225
Burtin P, Brunelle JP, Pijolat M, Soustelle M (1987) Appl Catal 34:239
Sehested J, Gelten JAP, Remediakis IN, Bengaard H, Nørskov JK (2004) J Catal 223:432
Schulze K, Makowski W, Chyży R, Dziembaj R, Geismar G (2001) Appl Clay Sci 18:59
Nurunnabi M, Mukainakano Y, Kado S, Miyazawa T, Okumura K, Miyao T, Naito S, Suzuki K, Fujimoto KI, Kunimori K, Tomishige K (2006) Appl Catal A 308:1
Wang SB, Lu GQM (1998) Appl Catal B 16:269
Dissanayake D, Rosynek MO, Kharras KCC, Lunsford JH (1991) J Catal 132:117
Zhang ZL, Verykios XE (1994) Catal Today 21:589
Chen L, Lua Y, Hong Q, Lin J, Dautzenberg FM (2005) Appl Catal A 292:295
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Qiu, Y., Chen, J. & Zhang, J. Effect of Calcination Temperature on Properties of Eggshell Ni/MgO–Al2O3 Catalyst for Partial Oxidation of Methane to Syngas. Catal Lett 127, 312–318 (2009). https://doi.org/10.1007/s10562-008-9680-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10562-008-9680-5