Abstract
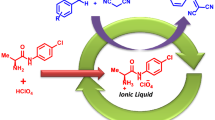
The novel efficient procedure has been developed for the oxathioacetalization of carbonyl compounds and 2-mercaptoethanol at room temperature under solvent-free condition. The results showed that the novel multi-SO3H functionalized ionic liquid was very efficient for the reactions with the good to excellent yields in short time. Operational simplicity, without need of any solvent, room temperature, low cost of the catalyst used, high yields, applicability to large-scale reactions, reusability and chemoselectivity are the key features of this methodology.




Similar content being viewed by others
References
Anastas PT, Kirchhoff MM (2002) Acc Chem Res 35:685
DeSimone JM (2002) Science 297:799
Harton B (1999) Nature 400:797
Batool A, Sedigheh T, Mozaffar A, Elham S (2006) J Porphyrins Phthalocyanines 10:167
Habib F, Nasser I, Abbas Ali J, Reza JM (2006) J Mol Catal A Chem 247:14
Khan AT, Parvin T, Choudhury LH (2006) Synthesis 2497
Chandrasekhar S, Jaya Prakash S, Shyamsunder T, Ramachandar T (2005) Synth Commun 35:3127
Ghanashyam B, Nabajyoti B (2006) Chem Lett 35:542
Bandgar BP, Kamble VT, Kulkarni A (2005) Aust J Chem 58:607
Kumar A, Jain N, Rana S, Chauhan SMS (2004) Synlett 2785
Aoyama T, Takido T, Kodomari M (2004) Synlett 2307
Khan AT, Sahu PR, Majee A (2005) J Mol Catal A Chem 226:207
Gogoi S, Borah JC, Barua NC (2004) Synlett 1592
Rana KK, Guin C, Jana S, Roy SC (2003) Tetrahedron Lett 44:8597
Kamal A, Chouhan G, Ahmed K (2002) Tetrahedron Lett 43:6947
Kazahaya K, Hamada N, Ito S, Sato T (2002) Synlett 1535
Mondal E, Sahu PR, Khan AT (2002) Synlett 463
Battaglia L, Pinna F, Strukul G (2001) Can J Chem 79:621
Perio B, Hamelin J (2000) Green Chem 2:252
Ravindranathan T, Chavan SP, Dantale SW (1995) Tetrahedron Lett 36:2285
Bergmann ED, Lavie D, Pinchas S (1951) J Am Chem Soc 73:5662
Acknowledgment
This work was supported by National Key Project of Scientific and Technical Supporting Programs Funded by Ministry of Science & Technology of China (No. 2006BAE03B06), Shanghai Leading Academic Discipline Project, Project Number: B409 and Shanghai International Cooperation of Science and Technology Project, Project Number: 06SR07101.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Liang, X., Gao, S., Yang, J. et al. Synthesis of a Novel Strong Brønsted Acidic Ionic Liquid and its Catalytic Activities for the Oxathioacetalization. Catal Lett 125, 396–400 (2008). https://doi.org/10.1007/s10562-008-9575-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10562-008-9575-5