Abstract
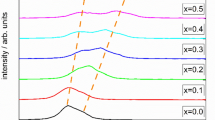
The bulk structure was investigated for Fe-based perovskite-type oxides with the formula La0.6Sr0.4CoyFe1−yO3−δ (y = 0.1, 0.2, and 0.3). The materials were confirmed to be stoichiometric with respect to oxygen under ambient conditions and the structural features were then further characterized under different environments as a function of temperature. Under reducing atmospheres, the degree of reduction increased with Co content, suggesting the presence of preferential oxidation of Fe over Co. Under milder conditions, oxygen vacancy formation was not proportional to Co content, which was likely caused by an electronic structure transition. The unit cell parameters were also shown to strongly depend upon Co content, temperature, and environment. A rhombohedral to cubic transition occurred at lower temperatures for higher Co content, but showed less dependence upon environment. A change in the thermal expansion behavior occurred at the temperature where oxygen vacancies formed leading to two regions of linear thermal expansion. The use of lattice parameters compared to dilatometry allowed for the simultaneous monitoring of unit cell symmetry and expansion behavior so the link between thermal properties and unit cell symmetry could be firmly established.












Similar content being viewed by others
References
Adler SB (2004) Chem Rev 104:4791
Mizusaki J, Yoshihiro M, Yamauchi S, Fueki K (1985) J Solid State Chem 58:257
Mizusaki J, Mima Y, Yamauchi S, Fueki K, Tagawa H (1989) J Solid State Chem 80:102
Mizusaki J, Moir N, Takai H, Yonemura Y, Minamiue H, Tagawa H, Dokiya M, Inaba H, Naraya K, Sasamoto T, Hashimoto T (2000) Solid State Ionics 129:163
Stevenson JW, Armstrong TR, Carneim RD, Pederson LR, Weber WJ (1996) J Electrochem Soc 143:2722
Tai LW, Nasrallah MM, Anderson HU, Sparlin DM, Sehlin SR (1995) Solid State Ionics 76:259
Tai LW, Nasrallah MM, Anderson HU, Sparlin DM, Sehlin SR (1995) Solid State Ionics 76:273
Lankhorst MHR, ten Elshof JE (1997) J Solid State Chem 130:302
Pena MA, Fierro JLG (2001) Chem Rev 101:1981
Wang S, Katsuki M, Dokiya M, Hashimoto T (2003) Solid State Ionics 159:71
Petrov AN, Kononchuk OF, Andreev AV, Cerepanov VA, Kofstad P (1995) Solid State Ionics 80:189
Wiik K, Aasland S, Hansen HL, Tangen IL, Ødegard R (2002) Solid State Ionics 152–153:675
Qui L, Lee TH, Liu LM, Yang YL, Jacobson AJ (1995) Solid State Ionics 76:321
Kruidhof H, Bouwmeester HJM, van Doorn RHE, Burggraaf AJ (1993) Solid State Ionics 63–65:816
McIntosh S, Vente JF, Haije WG, Blank DHA, Bouwmeester HJM (2006) Solid State Ionics 177:1737
Vente JF, McIntosh S, Haije WG, Bouwmeester HJM (2006) J Electrochem Soc 10:581
Yang ZH, Lin YS (2005) Solid State Ionics 176:89
Nemeth Z, Homonnay Z, Arva F, Klencsar Z, Kuzmann E, Hakl J, Vad K, Meszaros S, Kellner K, Gritzner G, Vertes A (2007) J Radioanal Nucl Chem 271:11
Petric A, Huang P, Tietz F (2000) Solid State Ionics 135:719
Adler SB (2001) J Am Ceram Soc 84:2117
Chen X, Yu J, Adler SB (2005) Chem Mater 17:4537
Yamazoe N, Teraoka Y, Seiyama T (1981) Chem Lett 1767
Seiyama T, Yamazoe N, Eguchi K (1985) Ind Eng Chem Prod Res Dev 24:19
Kaliaguine S, Van Neste A, Szabo V, Gallot JE, Bassir M, Muzychuk R (2001) Appl Catal A: Gen 209:345
Mantzavinos D, Hartley A, Metcalfe IS, Sahibzada M (2000) Solid State Ionics 134:103
Merino NA, Barbero BP, Ruiz P, Cadus LE (2006) J Catal 240:245
Kuhn JN, Ozkan US (2007) J Catal (accepted)
Acknowledgments
The financial support provided for this work by the Ohio Coal Development Office and the Ohio Department of Development through a Wright Center of Innovation is gratefully acknowledged. The authors also thank Rick B. Watson for technical assistance in the initial stages of this research.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kuhn, J.N., Ozkan, U.S. Effect of Co Content Upon the Bulk Structure of Sr- and Co-doped LaFeO3 . Catal Lett 121, 179–188 (2008). https://doi.org/10.1007/s10562-007-9364-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10562-007-9364-6