Abstract
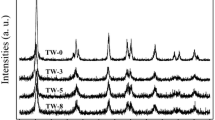
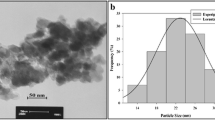
A composite oxide ZnAl2O4 was prepared by microwave-assisted hydrothermal treatment, a precursor mixture of hydroxides obtained by precipitation of aluminium and zinc nitrates. Characterization by TEM, XRD and textural studies shows that ZnAl2O4 is nanosized and is a micro/mesoporous material with large a surface area (140 m2/g). The gas phase catalytic methylation of 4-hydroxypyridine in the presence of the ZnAl2O4 catalyst was performed in a continuous process at atmospheric pressure in the temperature range of 240–360 °C. A mixture of O- and N-alkylated products, namely 4-methoxypyridine and N-methyl-4-pyridone were obtained. The alkylation of 4-hydroxypyridine with methanol at 345 °C offered 87.6% selectivity towards N-methyl-4-pyridone with about 89% 4-hydroxypyridine conversion.








Similar content being viewed by others
References
Electronic Medicines Compendium http://emc.medicines.org.uk/emc/assets/c/html/DisplayDoc.asp?DocumentID=6380
Stetinova V, Grossmann V (2000) Exp Toxicol Pathol 52:473
Dyer DJ, Lee VY, Twieg RJ (1997) Liquid Cryst 23:551
You F, Twieg RJ (1999) Tetrahedron Lett 40:8759
Gibson D, Clarke D, Winscom Ch (2000) US Pat 6,841,344
Grabowska H, Zawadzki M, Syper L (2006) Appl Catal A: Gen 314:226
Komarneni S, Katsuki H (2002) Pure Appl Chem 74:1537
Rao KJ, Mahesh K, Kumar S (2005) Bull Mater Sci 28:19
Malinger KA, Laubernds K, Son YC, Suib SL (2004) Chem Mater 16:4296
Ren ZW, Zhou DB, Tu SQ (2007) Chin J Catal 8:217
International Centre for Diffraction Date, ICDD, PDF-4 + 2005
Dollimore D, Heal GR (1964) J Appl Chem 14:109
Wrzyszcz J, Zawadzki M, Trawczyński J, Grabowska H, Miśta W (2001) Appl Catal A: Gen 210:263
Grabowska H, Miśta W, Trawczyński J, Wrzyszcz J, Zawadzki M (2001) Appl Catal A: Gen 220:207
Grabowska H, Zawadzki M, Syper L, Miśta W (2005) Appl Catal A: Gen 292:208
Sparks AK (1972) US Patent 3,670,030
Leach BE (1980) US Patent 4,227,024
IUPAC Recommendations (1994) Pure Appl Chem 66:1739
Di Cosimo JI, Diez VK, Xu M, Iglesia E, Apesteguia CR (1998) J Catal 178:499
Di Cosimo JI, Apesteguia CR, Gines MJL, Iglesia E (2000) J Catal 190:261
Beak P, Bonham J, Lee TJ Jr (1968) J Am Chem Soc 90:1569
Acknowledgments
The authors are very grateful to Mrs Ludwina Krajczyk for HRTEM studies and to Prof. Janusz Trawczyński for his help in acidity measurements.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Grabowska, H., Zawadzki, M. & Syper, L. Catalytic Method for N-Methyl-4-pyridone Synthesis in the Presence of ZnAl2O4 . Catal Lett 121, 103–110 (2008). https://doi.org/10.1007/s10562-007-9305-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10562-007-9305-4