Abstract
Purpose
Advancing age is the major risk factor for thoracic aortic aneurysm/dissection (TAAD). However, the causative link between age-related molecules and TAAD remains elusive. Here, we investigated the role of Sirtuin 1 (SIRT1, also known as class III histone deacetylase), the best studied member of the longevity-related Sirtuin family, in TAAD development in vivo.
Methods
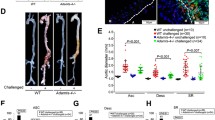
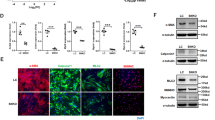
We used male smooth muscle-specific SIRT1 transgenic (ST-Tg) mice, smooth muscle-specific SIRT1 knockout (ST-KO) mice, and their wild-type (WT) littermates on a C57BL/6J background to establish a TAAD model induced by oral administration of 3-aminopropionitrile fumarate (BAPN). We analyzed the incidence and fatality rates of TAAD in the groups. We examined matrix metallopeptidase 2 (MMP2) and MMP9 expression in aortas or cultured A7r5 cells via western blotting and real-time polymerase chain reaction (PCR). We performed chromatin immunoprecipitation (ChIP) to clarify the epigenetic mechanism of SIRT1-regulated MMP2 expression in vascular smooth muscle cells (VSMCs).
Results
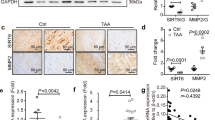
BAPN treatment markedly increased the incidence of TAAD in WT mice but caused less disease in ST-Tg mice. Moreover, ST-KO mice had the highest BAPN-induced TAAD fatality rate of all the groups. Mechanistically, SIRT1 overexpression resulted in lower MMP2 and MMP9 expression after BAPN treatment in both mouse aortas and cultured A7r5 cells. The downregulation of BAPN-induced MMP2 expression by SIRT1 was mediated by deacetylation of histone H3 lysine 9 (H3K9) on the Mmp2 promoter in the A7r5 cells.
Conclusion
Our findings suggest that SIRT1 expression in SMCs protects against TAAD and could be a novel therapeutic target for TAAD management.





Similar content being viewed by others
References
Luo F, Zhou XL, Li JJ, Hui RT. Inflammatory response is associated with aortic dissection. Ageing Res Rev. 2009;8(1):31–5. doi:S1568–1637(08)00032–9. https://doi.org/10.1016/j.arr.2008.08.001.
Chun AS, Elefteriades JA, Mukherjee SK. Medical treatment for thoracic aortic aneurysm - much more work to be done. Prog Cardiovasc Dis. 2013;56(1):103–8. doi:S0033–0620(13)00108–4. https://doi.org/10.1016/j.pcad.2013.05.008.
Goldfinger JZ, Halperin JL, Marin ML, Stewart AS, Eagle KA, Fuster V. Thoracic aortic aneurysm and dissection. J Am Coll Cardiol. 2014;64(16):1725–39. doi:S0735–1097(14)06008–2. https://doi.org/10.1016/j.jacc.2014.08.025.
LeMaire SA, Russell L. Epidemiology of thoracic aortic dissection. Nat Rev Cardiol. 2011;8(2):103–13. https://doi.org/10.1038/nrcardio.2010.187.
Elefteriades JA, Farkas EA. Thoracic aortic aneurysm clinically pertinent controversies and uncertainties. J Am Coll Cardiol. 2010;55(9):841–57. doi:S0735–1097(09)04075–3. https://doi.org/10.1016/j.jacc.2009.08.084.
Gillis E, Van Laer L, Loeys BL. Genetics of thoracic aortic aneurysm: at the crossroad of transforming growth factor-beta signaling and vascular smooth muscle cell contractility. Circ Res. 2013;113(3):327–40. https://doi.org/10.1161/CIRCRESAHA.113.300675.
Quintana RA, Taylor WR. Cellular mechanisms of aortic aneurysm formation. Circ Res. 2019;124(4):607–18. https://doi.org/10.1161/CIRCRESAHA.118.313187.
Raffetto JD, Khalil RA. Matrix metalloproteinases and their inhibitors in vascular remodeling and vascular disease. Biochem Pharmacol. 2008;75(2):346–59. https://doi.org/10.1016/j.bcp.2007.07.004.
McNulty M, Spiers P, McGovern E, Feely J. Aging is associated with increased matrix metalloproteinase-2 activity in the human aorta. Am J Hypertens. 2005;18(4 Pt 1):504–9. doi:S0895–7061(04)01150–1. https://doi.org/10.1016/j.amjhyper.2004.11.011.
Longo GM, Xiong W, Greiner TC, Zhao Y, Fiotti N, Baxter BT. Matrix metalloproteinases 2 and 9 work in concert to produce aortic aneurysms. J Clin Invest. 2002;110(5):625–32. https://doi.org/10.1172/JCI15334.
Xiong W, Knispel RA, Dietz HC, Ramirez F, Baxter BT. Doxycycline delays aneurysm rupture in a mouse model of Marfan syndrome. J Vasc Surg. 2008;47(1):166–72; discussion 72. doi:S0741–5214(07)01455–3. https://doi.org/10.1016/j.jvs.2007.09.016.
Chung AW, Yang HH, Radomski MW, van Breemen C. Long-term doxycycline is more effective than atenolol to prevent thoracic aortic aneurysm in marfan syndrome through the inhibition of matrix metalloproteinase-2 and -9. Circ Res. 2008;102(8):e73–85. https://doi.org/10.1161/CIRCRESAHA.108.174367.
Kida Y, Goligorsky MS. Sirtuins, cell senescence, and vascular aging. Can J Cardiol. 2016;32(5):634–41. doi:S0828-282X(15)01641–4. https://doi.org/10.1016/j.cjca.2015.11.022.
Yamamoto H, Schoonjans K, Auwerx J. Sirtuin functions in health and disease. Mol Endocrinol. 2007;21(8):1745–55. https://doi.org/10.1210/me.2007-0079.
Michan S, Sinclair D. Sirtuins in mammals: insights into their biological function. Biochem J. 2007;404(1):1–13. https://doi.org/10.1042/BJ20070140.
Guarente L, Picard F. Calorie restriction--the SIR2 connection. Cell. 2005;120(4):473–82. doi:S0092–8674(05)00103–0. https://doi.org/10.1016/j.cell.2005.01.029.
Lavu S, Boss O, Elliott PJ, Lambert PD. Sirtuins--novel therapeutic targets to treat age-associated diseases. Nat Rev Drug Discov. 2008;7(10):841–53. https://doi.org/10.1038/nrd2665.
Bordone L, Guarente L. Calorie restriction, SIRT1 and metabolism: understanding longevity. Nat Rev Mol Cell Biol. 2005;6(4):298–305. https://doi.org/10.1038/nrm1616.
Cohen HY, Miller C, Bitterman KJ, Wall NR, Hekking B, Kessler B, et al. Calorie restriction promotes mammalian cell survival by inducing the SIRT1 deacetylase. Science. 2004;305(5682):390–2. https://doi.org/10.1126/science.10991961099196.
Yu W, Fu YC, Chen CJ, Wang X, Wang W. SIRT1: a novel target to prevent atherosclerosis. J Cell Biochem. 2009;108(1):10–3. https://doi.org/10.1002/jcb.22240.
Zhang QJ, Wang Z, Chen HZ, Zhou S, Zheng W, Liu G, et al. Endothelium-specific overexpression of class III deacetylase SIRT1 decreases atherosclerosis in apolipoprotein E-deficient mice. Cardiovasc Res. 2008;80(2):191–9. https://doi.org/10.1093/cvr/cvn224.
Gao P, Xu TT, Lu J, Li L, Xu J, Hao DL, et al. Overexpression of SIRT1 in vascular smooth muscle cells attenuates angiotensin II-induced vascular remodeling and hypertension in mice. J Mol Med (Berl). 2014;92(4):347–57. https://doi.org/10.1007/s00109-013-1111-4.
Miyazaki R, Ichiki T, Hashimoto T, Inanaga K, Imayama I, Sadoshima J, et al. SIRT1, a longevity gene, downregulates angiotensin II type 1 receptor expression in vascular smooth muscle cells. Arterioscler Thromb Vasc Biol. 2008;28(7):1263–9. https://doi.org/10.1161/ATVBAHA.108.166991.
Chen HZ, Wang F, Gao P, Pei JF, Liu Y, Xu TT, et al. Age-associated sirtuin 1 reduction in vascular smooth muscle links vascular senescence and inflammation to abdominal aortic aneurysm. Circ Res. 2016;119(10):1076–88. https://doi.org/10.1161/CIRCRESAHA.116.308895.
Zhou S, Chen HZ, Wan YZ, Zhang QJ, Wei YS, Huang S, et al. Repression of P66Shc expression by SIRT1 contributes to the prevention of hyperglycemia-induced endothelial dysfunction. Circ Res. 2011;109(6):639–48. https://doi.org/10.1161/CIRCRESAHA.111.243592.
Kume S, Uzu T, Kashiwagi A, Koya D. SIRT1, a calorie restriction mimetic, in a new therapeutic approach for type 2 diabetes mellitus and diabetic vascular complications. Endocr Metab Immune Disord Drug Targets. 2010;10(1):16–24 doi:EMID-DT-ABS-22.
Oka S, Alcendor R, Zhai P, Park JY, Shao D, Cho J, et al. PPARalpha-Sirt1 complex mediates cardiac hypertrophy and failure through suppression of the ERR transcriptional pathway. Cell Metab. 2011;14(5):598–611. doi:S1550–4131(11)00385–8. https://doi.org/10.1016/j.cmet.2011.10.001.
Vikram A, Lewarchik CM, Yoon JY, Naqvi A, Kumar S, Morgan GM, et al. Sirtuin 1 regulates cardiac electrical activity by deacetylating the cardiac sodium channel. Nat Med. 2017;23(3):361–7. https://doi.org/10.1038/nm.4284.
Jia LX, Zhang WM, Zhang HJ, Li TT, Wang YL, Qin YW, et al. Mechanical stretch-induced endoplasmic reticulum stress, apoptosis and inflammation contribute to thoracic aortic aneurysm and dissection. J Pathol. 2015;236(3):373–83. https://doi.org/10.1002/path.4534.
Allaire E, Muscatelli-Groux B, Guinault AM, Pages C, Goussard A, Mandet C, et al. Vascular smooth muscle cell endovascular therapy stabilizes already developed aneurysms in a model of aortic injury elicited by inflammation and proteolysis. Ann Surg. 2004;239(3):417–27 doi:00000658–200403000-00018.
Visse R, Nagase H. Matrix metalloproteinases and tissue inhibitors of metalloproteinases: structure, function, and biochemistry. Circ Res. 2003;92(8):827–39. https://doi.org/10.1161/01.RES.0000070112.80711.3D92/8/827.
Pyo R, Lee JK, Shipley JM, Curci JA, Mao D, Ziporin SJ, et al. Targeted gene disruption of matrix metalloproteinase-9 (gelatinase B) suppresses development of experimental abdominal aortic aneurysms. J Clin Invest. 2000;105(11):1641–9. https://doi.org/10.1172/JCI8931.
Li L, Zhang HN, Chen HZ, Gao P, Zhu LH, Li HL, et al. SIRT1 acts as a modulator of neointima formation following vascular injury in mice. Circ Res. 2011;108(10):1180–9. https://doi.org/10.1161/CIRCRESAHA.110.237875.
Ryall JG, Dell'Orso S, Derfoul A, Juan A, Zare H, Feng X, et al. The NAD(+)-dependent SIRT1 deacetylase translates a metabolic switch into regulatory epigenetics in skeletal muscle stem cells. Cell Stem Cell. 2015;16(2):171–83. doi:S1934–5909(14)00562–1. https://doi.org/10.1016/j.stem.2014.12.004.
Taylor GC, Eskeland R, Hekimoglu-Balkan B, Pradeepa MM, Bickmore WA. H4K16 acetylation marks active genes and enhancers of embryonic stem cells, but does not alter chromatin compaction. Genome Res. 2013;23(12):2053–65. https://doi.org/10.1101/gr.155028.113.
Liu Y, Wang TT, Zhang R, Fu WY, Wang X, Wang F, et al. Calorie restriction protects against experimental abdominal aortic aneurysms in mice. J Exp Med. 2016;213(11):2473–88. https://doi.org/10.1084/jem.20151794.
Anzai A, Shimoda M, Endo J, Kohno T, Katsumata Y, Matsuhashi T, et al. Adventitial CXCL1/G-CSF expression in response to acute aortic dissection triggers local neutrophil recruitment and activation leading to aortic rupture. Circ Res. 2015;116(4):612–23. https://doi.org/10.1161/CIRCRESAHA.116.304918.
Kurihara T, Shimizu-Hirota R, Shimoda M, Adachi T, Shimizu H, Weiss SJ, et al. Neutrophil-derived matrix metalloproteinase 9 triggers acute aortic dissection. Circulation. 2012;126(25):3070–80. https://doi.org/10.1161/CIRCULATIONAHA.112.097097.
M-A M, G-R AB, G D, C E, M F, A A, et al. Deficiency of MMP17/MT4-MMP proteolytic activity predisposes to aortic aneurysm in mice. Circ Res. 2015;117(2):e13–26. https://doi.org/10.1161/circresaha.117.305108.
T BC, L C, S H, L W, R A, J X, et al. An adventitial IL-6/MCP1 amplification loop accelerates macrophage-mediated vascular inflammation leading to aortic dissection in mice. J Clin Invest. 2009;119(12):3637–51. https://doi.org/10.1172/jci38308.
Hellenthal FA, Buurman WA, Wodzig WK, Schurink GW. Biomarkers of AAA progression. Part 1: extracellular matrix degeneration. Nat Rev Cardiol. 2009;6(7):464–74. https://doi.org/10.1038/nrcardio.2009.80.
Hellenthal FA, Buurman WA, Wodzig WK, Schurink GW. Biomarkers of abdominal aortic aneurysm progression. Part 2: inflammation. Nat Rev Cardiol. 2009;6(8):543–52. https://doi.org/10.1038/nrcardio.2009.102.
Basatemur GL, Jorgensen HF, Clarke MCH, Bennett MR, Mallat Z. Vascular smooth muscle cells in atherosclerosis. Nat Rev Cardiol. 2019;16(12):727–44. https://doi.org/10.1038/s41569-019-0227-910.
Hu C, Tan H, Lin Q, Abudupataer M, Zhao Y, Li J, et al. SPECT/CT imaging of apoptosis in aortic aneurysm with radiolabeled duramycin. Apoptosis. 2019;24(9–10):745–55. https://doi.org/10.1007/s10495-019-01554-8.
Rowe VL, Stevens SL, Reddick TT, Freeman MB, Donnell R, Carroll RC, et al. Vascular smooth muscle cell apoptosis in aneurysmal, occlusive, and normal human aortas. J Vasc Surg. 2000;31(3):567–76 doi:S0741–5214(00)90319–7.
Thompson RW, Liao S, Curci JA. Vascular smooth muscle cell apoptosis in abdominal aortic aneurysms. Coron Artery Dis. 1997;8(10):623–31.
An Z, Liu Y, Song ZG, Tang H, Yuan Y, Xu ZY. Mechanisms of aortic dissection smooth muscle cell phenotype switch. J Thorac Cardiovasc Surg. 2017;154(5):1511–21 e6. doi:S0022–5223(17)31119–4. https://doi.org/10.1016/j.jtcvs.2017.05.066.
Liu W, Wang B, Wang T, Liu X, He X, Liu Y, et al. Ursodeoxycholic acid attenuates acute aortic dissection formation in angiotensin II-infused apolipoprotein E-deficient mice associated with reduced ROS and increased Nrf2 levels. Cell Physiol Biochem. 2016;38(4):1391–405. https://doi.org/10.1159/000443082.
North BJ, Verdin E. Sirtuins: Sir2-related NAD-dependent protein deacetylases. Genome Biol. 2004;5(5):224. https://doi.org/10.1186/gb-2004-5-5-224gb-2004-5-5-224.
Funding
This work was funded by grants from the National Natural Science Foundation of China (Numbers 81700411 and 91639110).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of Interest
The authors declare that they have no conflicts of interest.
Ethical Approval
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Wang, F., Tu, Y., Gao, Y. et al. Smooth Muscle Sirtuin 1 Blocks Thoracic Aortic Aneurysm/Dissection Development in Mice. Cardiovasc Drugs Ther 34, 641–650 (2020). https://doi.org/10.1007/s10557-020-07005-w
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10557-020-07005-w