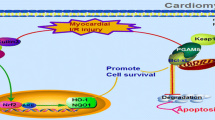
Abstract
Purpose
Necroptosis is an important form of cell death following myocardial ischemia/reperfusion (I/R) and phosphoglycerate mutase 5 (PGAM5) functions as the convergent point for multiple necrosis pathways. This study aims to investigate whether inhibition of PGAM5 could reduce I/R-induced myocardial necroptosis and the underlying mechanisms.
Methods
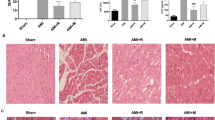
The SD rat hearts (or H9c2 cells) were subjected to 1-h ischemia (or 10-h hypoxia) plus 3-h reperfusion (or 4-h reoxygenation) to establish the I/R (or H/R) injury model. The myocardial injury was assessed by the methods of biochemistry, H&E (hematoxylin and eosin), and PI/DAPI (propidium iodide/4′,6-diamidino-2-phenylindole) staining, respectively. Drug interventions or gene knockdown was used to verify the role of PGAM5 in I/R (or H/R)-induced myocardial necroptosis and possible mechanisms.
Results
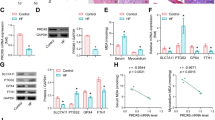
The I/R-treated heart showed the injuries (increase in infarct size and creatine kinase release), upregulation of PGAM5, dynamin-related protein 1 (Drp1), p-Drp1-S616, and necroptosis-relevant proteins (RIPK1/RIPK3, receptor-interacting protein kinase 1/3; MLKL, mixed lineage kinase domain-like); these phenomena were attenuated by inhibition of PGAM5 or RIPK1. In H9c2 cells, H/R treatment elevated the levels of PGAM5, RIPK1, RIPK3, MLKL, Drp1, and p-Drp1-S616 and induced mitochondrial dysfunctions (elevation in mitochondrial membrane potential and ROS level) and cellular necrosis (increase in LDH release and the ratio of PI+/DAPI+ cells); these effects were blocked by inhibition or knockdown of PGAM5.
Conclusions
Inhibition of PGAM5 can reduce necroptosis in I/R-treated rat hearts through suppression of Drp1; there is a positive feedback between RIPK1 and PGAM5, and PGAM5 might serve as a novel therapeutic target for prevention of myocardial I/R injury.







Similar content being viewed by others
References
He X, Li S, Liu B, Susperreguy S, Formoso K, Yao J, et al. Major contribution of the 3/6/7 class of TRPC channels to myocardial ischemia/reperfusion and cellular hypoxia/reoxygenation injuries. Proc Natl Acad Sci U S A. 2017;114(23):E4582–e91.
Harisseh R, Pillot B, Gharib A, Augeul L, Gallo-Bona N, Ferrera R, et al. Unacylated ghrelin analog prevents myocardial reperfusion injury independently of permeability transition pore. Basic Res Cardiol. 2017;112(1):4.
Yang C, Liu X, Yang F, Zhang W, Chen Z, Yan D, et al. Mitochondrial phosphatase PGAM5 regulates Keap1-mediated Bcl-xL degradation and controls cardiomyocyte apoptosis driven by myocardial ischemia/reperfusion injury. In vitro cellular & developmental biology. Animal. 2017;53(3):248–57.
Orrenius S, Zhivotovsky B, Nicotera P. Regulation of cell death: the calcium-apoptosis link. Nature reviews. Mol Cell Biol. 2003;4(7):552–65.
Elmore S. Apoptosis: a review of programmed cell death. Toxicol Pathol. 2007;35(4):495–516.
Adameova A, Goncalvesova E, Szobi A, Dhalla NS. Necroptotic cell death in failing heart: relevance and proposed mechanisms. Heart Fail Rev. 2016;21(2):213–21.
Fietta P. Many ways to die: passive and active cell death styles. Riv Biol. 2006;99(1):69–83.
Ying Y, Padanilam BJ. Regulation of necrotic cell death: p53, PARP1 and cyclophilin D-overlapping pathways of regulated necrosis? Cell Mol Life Sci. 2016;73(11–12):2309–24.
Tonnus W, Linkermann A. The in vivo evidence for regulated necrosis. Immunol Rev. 2017;277(1):128–49.
Ashkenazi A, Salvesen G. Regulated cell death: signaling and mechanisms. Annu Rev Cell Dev Biol. 2014;30:337–56.
Vanden Berghe T, Linkermann A, Jouan-Lanhouet S, Walczak H, Vandenabeele P. Regulated necrosis: the expanding network of non-apoptotic cell death pathways. Nature reviews. Mol Cell Biol. 2014;15(2):135–47.
Conrad M, Angeli JP, Vandenabeele P, Stockwell BR. Regulated necrosis: disease relevance and therapeutic opportunities. Nature reviews. Drug Discov. 2016;15(5):348–66.
Grootjans S, Vanden Berghe T, Vandenabeele P. Initiation and execution mechanisms of necroptosis: an overview. Cell Death Differ. 2017;24(7):1184–95.
Linkermann A, Brasen JH, Darding M, Jin MK, Sanz AB, Heller JO, et al. Two independent pathways of regulated necrosis mediate ischemia-reperfusion injury. Proc Natl Acad Sci U S A. 2013;110(29):12024–9.
Hussain M, Zimmermann V, van Wijk SJL, Fulda S. Mouse lung fibroblasts are highly susceptible to necroptosis in a reactive oxygen species-dependent manner. Biochem Pharmacol. 2018;153:242–7.
Schreiber A, Rousselle A, Becker JU, von Massenhausen A, Linkermann A, Kettritz R. Necroptosis controls NET generation and mediates complement activation, endothelial damage, and autoimmune vasculitis. Proc Natl Acad Sci U S A. 2017;114(45):E9618–e25.
Qin D, Wang X, Li Y, Yang L, Wang R, Peng J, et al. MicroRNA-223-5p and -3p cooperatively suppress necroptosis in ischemic/reperfused hearts. J Biol Chem. 2016;291(38):20247–59.
Wang K, Liu F, Liu CY, An T, Zhang J, Zhou LY, et al. The long noncoding RNA NRF regulates programmed necrosis and myocardial injury during ischemia and reperfusion by targeting miR-873. Cell Death Differ. 2016;23(8):1394–405.
Oerlemans MI, Liu J, Arslan F, den Ouden K, van Middelaar BJ, Doevendans PA, et al. Inhibition of RIP1-dependent necrosis prevents adverse cardiac remodeling after myocardial ischemia-reperfusion in vivo. Basic Res Cardiol. 2012;107(4):270.
Wang JX, Zhang XJ, Li Q, Wang K, Wang Y, Jiao JQ, et al. MicroRNA-103/107 regulate programmed necrosis and myocardial ischemia/reperfusion injury through targeting FADD. Circ Res. 2015;117(4):352–63.
Wang Z, Jiang H, Chen S, Du F, Wang X. The mitochondrial phosphatase PGAM5 functions at the convergence point of multiple necrotic death pathways. Cell. 2012;148(1–2):228–43.
Chaikuad A, Filippakopoulos P, Marcsisin SR, Picaud S, Schroder M, Sekine S, et al. Structures of PGAM5 provide insight into active site plasticity and multimeric assembly. Structure (London, England: 1993). 2017;25(7):1089–99.e3.
He GW, Gunther C, Kremer AE, Thonn V, Amann K, Poremba C, et al. PGAM5-mediated programmed necrosis of hepatocytes drives acute liver injury. Gut. 2017;66(4):716–23.
Zhang YS, He L, Liu B, Li NS, Luo XJ, Hu CP, et al. A novel pathway of NADPH oxidase/vascular peroxidase 1 in mediating oxidative injury following ischemia-reperfusion. Basic Res Cardiol. 2012;107(3):266.
Chen S, Lv X, Hu B, Shao Z, Wang B, Ma K, et al. RIPK1/RIPK3/MLKL-mediated necroptosis contributes to compression-induced rat nucleus pulposus cells death. Apoptosis. 2017;22(5):626–38.
Shen C, Wang C, Han S, Wang Z, Dong Z, Zhao X, et al. Aldehyde dehydrogenase 2 deficiency negates chronic low-to-moderate alcohol consumption-induced cardioprotection possibly via ROS-dependent apoptosis and RIP1/RIP3/MLKL-mediated necroptosis. Biochim Biophys Acta. 2017;1863(8):1912–8.
Takahashi N, Duprez L, Grootjans S, Cauwels A, Nerinckx W, DuHadaway JB, et al. Necrostatin-1 analogues: critical issues on the specificity, activity and in vivo use in experimental disease models. Cell Death Dis. 2012;3:e437.
Welz PS, Wullaert A, Vlantis K, Kondylis V, Fernandez-Majada V, Ermolaeva M, et al. FADD prevents RIP3-mediated epithelial cell necrosis and chronic intestinal inflammation. Nature. 2011;477(7364):330–4.
Vandenabeele P, Grootjans S, Callewaert N, Takahashi N. Necrostatin-1 blocks both RIPK1 and IDO: consequences for the study of cell death in experimental disease models. Cell Death Differ. 2013;20(2):185–7.
Degterev A, Maki JL, Yuan J. Activity and specificity of necrostatin-1, small-molecule inhibitor of RIP1 kinase. Cell Death Differ. 2013;20(2):366.
Kang YJ, Bang BR, Han KH, Hong L, Shim EJ, Ma J, et al. Regulation of NKT cell-mediated immune responses to tumours and liver inflammation by mitochondrial PGAM5-Drp1 signalling. Nat Commun. 2015;6:8371.
Funding
This work was supported by the National Natural Science Foundation of China (No. 81872873 to Jun Peng; No. 81573430 to Xiu-Ju Luo; No. 81703516 to Bin Liu).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Ethical Approval
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic Supplementary Material
ESM 1
(DOC 1144 kb)
Rights and permissions
About this article
Cite this article
She, L., Tu, H., Zhang, YZ. et al. Inhibition of Phosphoglycerate Mutase 5 Reduces Necroptosis in Rat Hearts Following Ischemia/Reperfusion Through Suppression of Dynamin-Related Protein 1. Cardiovasc Drugs Ther 33, 13–23 (2019). https://doi.org/10.1007/s10557-018-06848-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10557-018-06848-8